Document Type : Original Article
Authors
1 Department of Anesthesiology and Critical Care, Arak University of Medical Sciences, Arak, Iran
2 Department of Infectious Disease, Arak University of Medical Sciences, Arak, Iran
3 Department of Cardiovascular Surgery, Arak University of Medical Science, Arak, Iran
Abstract
Background: Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has been associated with severe respiratory disease incidence and increased morbidity and mortality of patients all over the world. The aim of this study is evaluation of Atorvastatin effect on patients with COVID-19.
Materials and methods: This is a prospective randomized controlled trial with 100 adults diagnosed with COVID-19. Participants randomly received Atorvastatin or just routine protocol of treatment for 10 days. Primary outcomes are duration of fever and cough, atrial oxygen saturation (SPO2), length of hospitalization and ICU stay, duration of oxygen therapy or intubation, and mortality rate. Secondary outcomes contain white blood cell count, lymphocyte percentage, lactate dehydrogenase, and creatine phosphokinase level.
Results: There was no significant difference in age and sex between two groups. Atrial oxygen saturation (SPO2) with/without non-invasive oxygen therapy was significantly higher in statin group (P=0.001, P=0.002, respectively). However, other primary and secondary outcomes was similar in both groups.
Conclusion: Statin use was associated with higher percentage of SP02, but there were no significant differences in other outcomes.
Graphical Abstract
Keywords
Main Subjects
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has been associated with severe respiratory disease incidence and increased morbidity and mortality of patients all over the world [1, 2]. Coronavirus is an enveloped, positive-sense single-stranded RNA virus resulting in more than 2.9 million deaths [3]. In December 2019, the first case reported in China and on March 2020 COVID-19 was declared as a global pandemic by the World Health organ (WHO) and now more than 156 countries are affected. Clinical presentation of COVID-19 is various from asymptomatic to severe signs [4]. Significant clinical presentations of COVID-19 are fever, dry cough, dyspnea, and pneumonia. Likewise, anosmia and ageusia (smell and taste disorder), myalgia, weakness, and headache are common [5]. Identification of the virus structure and evaluation of its mechanism to the entrance to human cells can help to choose appropriate treatment. S protein on the surface of Coronavirus structure allows it to attach to angiotensin-converting enzyme 2 (ACE2) receptor by lipid rafts of plasma membrane so lipids and cholesterol of cell membrane are very important for the virus entrance [6]. Since ACE2 receptor is founded in various parts of the body such as the upper and lower airway, kidneys, myocardium, brain, and gastrointestinal tissues, coronavirus can induce multi-organ damages. Dysregulation of the immune system with an increase in cytokine release and endothelial damages due to entry of SARS-CoV2 into endothelial cells are some of the COVID-19 mechanisms [7]. Statin or HMG-CoA reductase inhibitor is famous for pleiotropic effects like anti-inflammatory, lipid-lowering, immunomodulatory, and anti-thrombotic, so it can be used for decreasing severity of COVID-19. Studies about statin effects in viral infections show that reduction of cholesterol in plasma membrane results lower viral entrance [8]. In addition, anti-inflammatory activity of statins might improve outcomes of severely ill patients due to decrease of D-dimer and interleukin-6 levels which are associated with mortality [9]. Whether there are some studies about statins and their beneficial effects on viral disease, the role of statins remains uncertain and clinical trial studies about the effect of statins on Coronaviridae are limited [9].
Furthermore, previous studies are about the association between antecedent statin use and COVID-19 severity. Hence, the aim of this prospective study is evaluation of hospital use of statin and its effect on the intensity of Clinical symptoms in patients with COVID-19.
Materials and Methods
This prospective randomized clinical trial involved two groups, the Statin group, and the control group, comprising patients with confirmed COVID-19 who were hospitalized at Amiralmoemenin Hospital in Arak City, Iran, between 13th March 2021 and 20th April 2021. The study was ethically approved with no. IR.ARAKMU.REC.1399.257 by the Ethical Committee of Arak University of Medical Science (IRCT20201028049175N3).
The inclusion criteria for this study were adults aged between 18 and 55 years, hospitalized with confirmed COVID-19 based on biological (polymerase chain reaction-PCR) and/or radiological findings, and who provided informed consent. Patients with history of long-term colchicine, cyclosporine, antifungal azoles, niacin and statin use, history of allergy to statins, chronic kidney disease (CKD), elevated Aspartate Transaminase (AST), and Alanine Transaminase (ALT) more than two times, elevated Creatine Phosphokinase (CPK) more than 5 times and pregnant or lactating females were excluded from the study.
The intervention was initiated just after confirming COVID-19 through a PCR test or chest CT scan. Patients without mechanical ventilation were prescribed orally administered Atorvastatin tablets with a daily dosage of 40 mg, while patients with invasive mechanical ventilation received Atorvastatin through gavage injection; this treatment was repeated every day. The control group was assigned a placebo (flour tablet) using the same method of administration as the statin group. The intervention lasted for 10 days or until death/discharge. Both groups received standard treatment based on the COVID-19 national protocol, and the use of routine medication was identified.
The dosage of Atorvastatin was standardized at 40 mg daily. Serious adverse events, including liver, renal, or muscle dysfunction were observed during the study. The benefits and potential risks were clearly outlined in the informed consent document.
Participants and investigators were unaware of the study and intervention grouping to avoid bias. Data collection and statistical analyses were conducted by investigators not involved in patient enrollment or treatment, while the clinical staff and investigators who enrolled and treated the patients were aware of the group assignments. To maintain blinding, Atorvastatin tablets and flour tablets were made similar in shape, size, and color, ensuring that participants could not distinguish between the treatments they received.
Demographic data of the patients were recorded on the first day of admission, and vital signs, respiratory indices (including ventilation parameters and oxygen saturation (SpO2)), white cell count, lymphocyte percentage, and lactate dehydrogenase levels were collected on the first and last days of admission. All data were collected from the hospital's clinical information system and recorded in checklist formats.
The study's outcomes focused on clinical presentations, such as the duration of febrile episodes and cough, the occurrence of hypoxia requiring supplemental oxygen or invasive mechanical ventilation, and intubation duration, length of ICU stay, hospitalization, and mortality.
Statistical significance was set at p < 0.05. All statistical tests were two-sided and performed using SPSS software, version 19, for analysis. Normality hypothesis was assessed using the Kolmogorov-Smirnov test, and parametric and non-parametric tests were used for normal and abnormal variables, respectively.
Results and Discussion
The present study included 1296 patients with COVID-19, comprising 557 women (43%) and 739 men (57%), with a mean age of 66.5 ± 15.4. There were no statistically significant differences between the two patient groups in terms of underlying characteristics, such as age and sex.
The statin group showed significantly higher mean oxygen saturation than the control group, as determined by the Mann-Whitney test. However, the levels of inflammatory markers, including LDH and WBC, were not significantly different between the statin and control groups (P-value = 0.98). The severity of patients' clinical symptoms, such as the duration of fever and cough, were similar in both groups (P = 0.54 and P = 0.80, respectively).
Furthermore, the duration of oxygen therapy and intubation in the statin group were observed to be shorter than in the control group, although no statistically significant differences were found between the two groups for these variables (P = 0.38 and P = 0.56, respectively). Likewise, the length of hospitalization and ICU stay, as well as the mortality rate, did not significantly differ between the two groups (P = 0.42, P = 0.68, and P = 0.65, respectively). Although the serum CPK (creatine phosphokinase) level was higher in the intervention group, this difference was not statistically significant when compared with the control group (P = 0.8) (Table 1 and 2).
In this prospective clinical trial, we investigated the association of statin use in hospitalized patients with COVID-19 with disease severity and mortality. The main finding of this study was that daily administration of 40 mg of statin in patients admitted with a definitive diagnosis of COVID-19 resulted in improved arterial oxygen saturation (SPO2,) but a link between the use of this drug and the improvement of other clinical symptoms in patients and their deaths were not found. In this regard, Timo tius et al. in a meta-analysis study involving 9 studies and 34449 patients, did not report an association between statin use and improvement of clinical symptoms in hospitalized patients with COVID-19 [10], but other studies have shown different results.
Table 1: Laboratory values and vital signs of patients included into the study
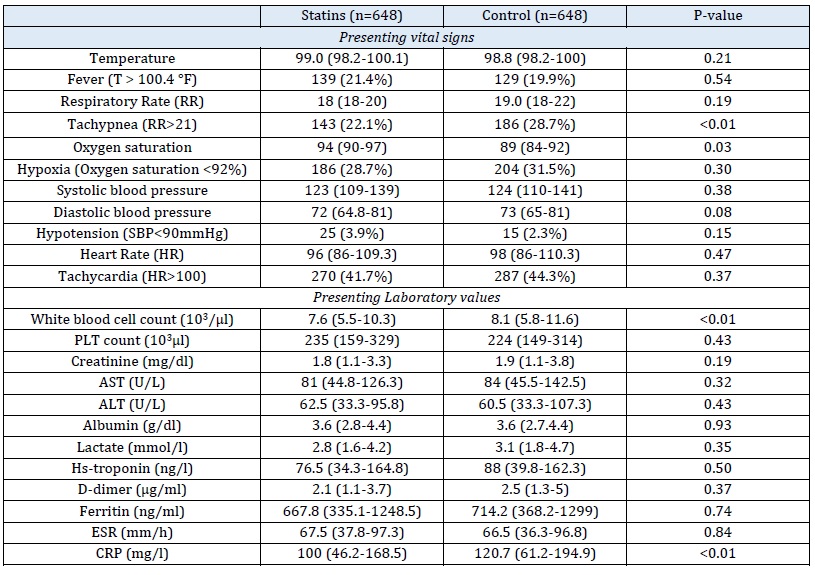
Table 2: Association of statin use with severe COVID-19 outcomes

In a study by Wilnord Y.T.Tan et al., they reported that statin use was independently associated with reduced ICU admission among COVID-19 patients [11]. In a study by Sophiat.Song et al., statin use was significantly associated with a reduced risk of invasive mechanical ventilation [12]. Another study conducted by Omar Saeed et al. investigated the effect of statins on the mortality of hospitalized diabetic patients with COVID-19, they found that despite the presence of more underlying diseases in the statin group, mortality from the COVID -19 disease was lower in this group (23%) than in the control group (27%) [13].
Jawad Haidar Butt et al. published a study in 2020 stating that recent statin uses in patients with Covid-19 was not associated with an increased or decreased risk of mortality or severe disease [14]. On the other hand, in a retrospective study conducted by Xiao-Jing Zhang et al., it has been reported that prescribing statins for patients with COVID-19 was associated with a reduced risk of death, but prospective RCT studies are needed to investigate this issue [15]. Other studies have reported similar results on the effect of statins in improving disease outcomes and mortality [16, 17]. The effect of statin as an inhibitor of HMG-CoA reductase enzyme has been proven to reduce lipids, which can reduce the lipid in the cell membrane as well as reduce its accumulation within the cell. SARS-CoV2 is capable of interacting with Angiotensin-converting enzyme 2 (ACE2) cell receptor due to its structure and the glycoprotein S at its surface. Since high fat (cholesterol) increases the accumulation of ACE2 receptors, SARS-CoV2 entry into the cell is expected to be higher in patients with high serum lipid levels. It should be noted that RNA viruses need cholesterol and intracellular fatty acids after entering the host cell to replicate. Therefore, atorvastatin with its fat-reducing properties can have a significant effect on reducing the entry of RNA viruses such as SARS-CoV2 into the cell and their proliferation [11].
Since statins also have pleiotropic effects such as immunomodulatory effect, improved vascular endothelial function, reduced inflammation and oxidative stress, they can play a role in improving the outcomes of patients with COVID-19 and the severity of the disease [14].
Statins are capable of improving the patient's condition and preventing the disease severity by stopping the molecular mechanisms that play a role in causing cytokine storms in patients with COVID-19 and exacerbating the disease (such as NF-KB, Toll-like receptor [TLR]-signaling), [18-20].
The possible effect of this class of drugs in patients with bacterial sepsis, pneumonia, and influenza has also been reported in previous studies [21, 22]. SARS-CoV2 directly and indirectly can lead to cardiovascular disorders through its effect on ACE2 function and impair vascular endothelial integrity, resulting in an increased chance of death in patients with COVID-19.
Therefore, statins with their cardiovascular-protective effect can help control these disorders [23, 24]. Despite all the theories about the capabilities of statins and their possible effects on the severity of symptoms and mortality in patients with COVID-19, studies have reported that a history of statin use in patients with COVID-19 was not linked to reduction of the severity of symptoms or mortality, while other studies have linked statin use to improving patient outcomes, reducing hospital stays, the need for mechanical ventilation, and patient mortality [10-14].
Most studies investigating the effect of statins on the outcomes of patients with COVID-19 have been retrospective and patient information has been extracted through a registry or electronic system. In addition, most studies have examined the effect of statins on the symptoms of COVID-19 in patients with a history of drug use. Moreover, few studies have evaluated the effect of statins in patients without history of taking statins and few studies have examined the effect of these drugs after confirming the diagnosis of COVID-19 during hospitalization (as RCT). Likewise, studies that were observational in nature could not assess the cause-effect. In addition, patients with a history of statin use have confounding factors, including the fact that statin users are often treated with anticoagulants such as aspirin due to other comorbid conditions, and this can be a confounding factor [25, 26].
Study limitations
The present study has some limitations that need to be considered. Although the authors tried to consider confounding factors as much as possible, cases such as the presence of comorbidities in patients and other medications that may be involved in the mechanism of action of statins were not available. Unfortunately, the lipid profile of patients before and after the study, which could be helpful in investigating the relationship between the effect of statins on serum lipid levels and the relationship between the two with the disease severity, was not measured in the laboratory. Other limitations in this study include the lack of study of other types of statins and the lack of comparison of different doses of this drug, which is suggested to be considered in future RCTs. Examining the use of this drug for a longer period may have better effects. The low sample size in this study can be one of the reasons that the differences between some variables in the two groups were not statistically significant, so it is recommended to perform similar RCTs with larger sample sizes.
Conclusion
These findings of this study suggest that while statin therapy may have a positive impact on oxygen saturation, it does not seem to significantly influence other critical outcomes in COVID-19 patients, such as inflammatory markers, clinical symptomatology, disease severity, or mortality.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
ORCID
Alireza Kamali
https://orcid.org/0000-0003-0698-340X
Behnam Mahmodiyeh
https://orcid.org/0000-0002-5554-6866
Masoumeh Sofian
https://orcid.org/0000-0002-8564-3659
Elham Farahani
https://orcid.org/0000-0002-7904-8247
Shamim Valibeik
https://orcid.org/0000-0003-3777-0909
HOW TO CITE THIS ARTICLE
Alireza Kamali, Masoumeh Sofian, Behnam Mahmodiyeh, Shamim Valibeik, Elham Farahani. Effect of Atorvastatin on Clinical Manifestations and Outcomes in Patients with COVID-19. J. Med. Chem. Sci., 2024, 7(1) 242-249.


