Document Type : Original Article
Authors
- Adeel Khalid 1
- Asma Asghar 1
- M. Danish 2
- Ayesha Ijaz 1
- Ammara Zahid 1
- Arif Nur Muhammad Ansori 3, 4, 5, 6
- Ahmad Affan Ali Murtadlo 3, 5, 6
- Rahadian Zainul 7, 8
1 Department of Zoology, University of Sialkot, Sialkot, Punjab, Pakistan
2 Department of Chemistry, University of Sialkot, Sialkot, Punjab, Pakistan
3 Faculty of Science and Technology, Universitas Airlangga, Surabaya, Indonesia
4 Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun, India
5 Division of Research and Development, CV Jalan Tengah, Pasuruan, Indonesia
6 Division of Molecular Biology and Genetics, Generasi Biologi Indonesia Foundation, Gresik, Indonesia
7 Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Padang, Padang, Indonesia
8 Center for Advanced Material Processing, Artificial Intelligence, and Biophysic Informatics (CAMPBIOTICS), Universitas Negeri Padang, Padang, Indonesia
Abstract
Titanium dioxide nanoparticles (TiO2-NPs) are widely utilized as a whitish pigment with potential applications in various sectors, including cosmetics, pharmaceuticals, food production, textiles, and water treatment. They are commonly employed as a sanitizing agent for microorganism control. Due to their small size and expansive surface area, these nanoparticles can inflict severe toxic effects on various human organs and cause serious damage to the surrounding environment. This study investigated the gonadal toxicity induced by TiO2-NPs in male Sprague Dawley rats. Accordingly, TiO2-NPs were employed with an average particle size of 55 nm. Twenty-five adult male Sprague Dawley rats weighing 107 ± 120 g underwent a 7-day acclimatization period and were subsequently randomly divided into five groups: The Control group (C), which received no treatment; the saline-treated group (S), treated with normal saline; and three treatment groups (G1, G2, and G3), each consisting of five rats. The rats were exposed to different concentrations of TiO2-NPs for 28 days on alternate days, determined after establishing the LC50 value. TiO2 was administered intraperitoneally with injection at doses of 50, 90, and 130 mg/kg body weight. The blood samples were collected at the beginning and end of the experiment. The 28-day exposure results revealed severe histological damage in testicular tissues at high and medium doses compared to the control and saline-treated groups. These damages included widened lumen, basement membrane damage, necrosis, and vacuolation in the tissues, which were highly significant in both the G1 and G2 groups. This study demonstrates the toxic effects of titanium dioxide nanoparticles on the gonads of Sprague Dawley rats. It is concluded that the selected concentrations of TiO2 negatively impact the health of living organisms.
Graphical Abstract
Keywords
Main Subjects
Introduction
Nanotechnology has drawn increasing commercial and scientific interest over the last decade due to its incredible features, which include optical characteristics, flexibility, reactivity, and improved strength. Consequently, nanoparticles find frequent applications in electronics, cosmetics, medicine, and antimicrobial agents [1]. Titanium dioxide nanoparticles (TiO2-NPs) are utilized, produced, and discarded worldwide, potentially leading to their release into the environment and causing potential damage to various organisms and ecosystems. Beyond their outstanding antibacterial properties, titanium dioxide (TiO2) nanoparticles are a critical component of skin care products such as sunblock, as they absorb the UV light and effectively shield the skin from harmful UV exposure [2].
Despite their economic benefits, their introduction into the environment may pose adverse biological consequences. Measuring the levels of various chemicals in various tissues and organs, in conjunction with fluctuating biochemical markers, which fluctuate depending on the nature of exposure and different doses, allows the estimation of animal exposure to TiO2-NPs [3].
Nanoparticles (NPs) can be either organic or inorganic materials within the size range of 1 to 100 nanometers. They can be synthesized through chemical, physical, or natural/green methods [4]. Inorganic nanoparticles offer a multitude of fantastic applications, but their induced nanotoxicity poses a significant societal problem. The substantial surface area-to-mass ratio, one of their most distinctive properties, exerts significant toxic effects due to the presence of highly reactive surface atoms. Titanium dioxide (TiO2) nanoparticles are among various inorganic nanomaterials identified in various industries, including textiles, plastics, skincare products, and food packaging. These NPs have been previously synthesized through various techniques, including physicochemical techniques such as microemulsion, various chemical precipitation techniques, hydrothermal crystallization, and sol-gel techniques [5, 6]. Titanium dioxide is a whitish pigment characterized by a high refractive index and excellent brightness. It is used in confections and various bakery products, such as sauces, cakes, and pastries.
Examination of food quality containing TiO2 revealed that up to 36 percent of the particles consisted of TiO2-NPs, with an average particle size of 110 nm or within the range of 30-400 nm. Commercial gums, according to a study, contain over 40% TiO2, which can be released and ingested when consumed.
These NPs are more detrimental than larger TiO2 particles despite bulk TiO2 being considered as an inactive and nontoxic substance.
Due to their reactive nature, TiO2-NPs exhibit extreme toxicity and adversely affect various internal organs within the body. TiO2NPs can accumulate in the hippocampal region of the brain, entering through the olfactory bulb, where they can induce oxidative stress and mitochondrial dysfunction. Furthermore, they have the capacity to modify the cellular structure of living organisms [7-10].
TiO2-NPs have detrimental effects on the brain, liver, kidneys, spleen, lungs, and, especially, the reproductive system in mice, among other organs [11-13]. In vitro studies conducted on Leydig cells have revealed that TiO2-NPs migrate to these cells, inhibit their survival and proliferation, and increase the levels of heme oxygenase-1 gene and the steroidogenic acute regulatory protein. When TiO2-NPs are intraperitoneally administered to male mice, they result in a higher frequency of aberrant sperm and germ cell death, accompanied by significant reductions in sperm count and motility [14, 15].
Various studies demonstrated that TiO2-NPs infiltrate mouse Leydig cells and male germ cells, leading to variations in gene expression. TiO2-NPs can enter the testes and the reproductive system, which are particularly vulnerable to nanoparticles [16]. These nanoparticles could pose harmful effects on the germ line, either directly or indirectly, and somatic nurse cells, which may lead to sterility (Braydich-Stolle et al., 2005). In addition, they can impair testicular function and disrupt spermatogenesis in male mice [17, 18]. TiO2-NPs can penetrate the testicular barrier through the bloodstream and accumulate within the testes, giving rise to testicular lesions, changes in serum sex hormone levels, and cell death [19]. Concerning the adverse effects of these nanoparticles, this study investigated the TiO2 toxicity. The present research was designed to assess the toxic effects of various sub-lethal doses of TiO2-NPs on the gonads and blood of male Sprague-Dawley rats through histological and hematological analysis of the testicular tissues of the rats [20].
Materials and Methods
This study was conducted at the Research Laboratory of Government College University, Faisalabad, specifically within the Department of Zoology, involving the synthesis of TiO2 nanoparticles, gonadal histology, and hematology. Ethical approval for this study was obtained from the Ethical Committee of the University of Sialkot, Pakistan.
Procurement of animals
A total of 25 healthy male post-weaning Sprague Dawley rats were obtained from the animal house of Government College University, Faisalabad, following approval from the ethical committee of the University of Sialkot. After a 7-day acclimatization period, rats of similar weight were sorted into five groups, each comprising five rats. These rats were placed in well-ventilated cages under standard lighting conditions and had unrestricted access to food and water.
TiO2-NPs experimental plan and sampling
The first group, designated as the control group (C), received a standard feed. The second group, referred to as the placebo group (P), was subjected to a subcutaneous injection of 1 ml of distilled water to simulate a subcutaneous shock. The remaining groups (G1, G2, and G3) were subcutaneously injected with TiO2 nanoparticles at doses of 50, 90, and 130 mg/kg body weight of the rats (Table 1). Blood samples from all of the animals were collected at the outset of the experiment, and after the treatment phase, these samples were analyzed following 28 days of hematological analysis, gonadal function testing, and histological alteration analysis. On the 29th day, following the collection of blood samples, the rats were euthanized, their gonadal tissues were harvested and weighed, and small tissue samples were immersed in 10% formalin for histological processing using the hematoxylin-eosin staining technique [21].
NPs characterization
Ninety-seven percent of TiO2 was supplied in white powder form by Sigma Aldrich Company and subjected to characterization using two methods: XRD (X-ray diffraction) and FTIR (Fourier transform infrared). XRD is a unique technique employed for structural analysis, offering insights into various characteristics, including crystallite size, structural analysis, impurity detection, lattice parameters, and crystal formation. This method is utilized to investigate materials in both solid and thin film forms, relying on Bragg's diffraction law (nλ = 2d sinΘ) [22].
FT-IR spectroscopy was employed to analyze the chemical structure of the carbon particles and identify the presence of any functional groups in the carbon nanoparticles. During the thermal decomposition of kerosene, additional compounds may form alongside carbon particles. According to reports, the combustion of kerosene in the atmosphere results in a complex mixture of elemental carbon, hydrocarbons, and other substances.
Table 1: Division of different treatment groups of rats
|
Group |
Treatment |
|
Control (C) |
No treatment |
|
Saline-treated group (S) |
(Normal saline injected intraperitoneally) |
|
G1 |
TiO2-NPs (intraperitoneally injected) @50mg/kg BW |
|
G2 |
TiO2-NPs (intraperitoneally injected) @90 mg/kg BW |
|
G3 |
TiO2-NPs (intraperitoneally injected) @130 mg/kg BW |
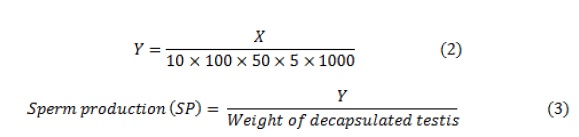
Where, X = Number of sperms counted in the Harwell chamber; Y = Number of spermatids in the homogenate; 10 = Number of squares recorded in one reading; Total squares in the chamber = 100.50 μL = Volume of homogenate solution for the chamber; 5 = Dilution factor with physiological saline; and 1,000 = Conversion factor from μL to mL.
Hematological analysis
Blood samples for CBC analysis were obtained by puncturing the caudal vein of each animal, and they were immediately placed in two tubes containing calcium EDTA. These samples were promptly used to estimate various hematological parameters, including HGB, WBC, RBC, LYM, MCV, MCH, PLT, and MCHC, using a hematology auto-analyzer. Furthermore, serum was collected from clotted blood samples to determine testosterone levels through the use of a TEIA kit [23].
Sperm count test
To conduct this procedure, a necessary technique was followed. The left testes of the treated rats were removed and stored in normal saline [24]. After removing the upper layer, known as the tunica albuginea, the weight of the testes was recorded and subsequently reweighed. The testes were homogenized in 50 mL of a homogenizing solution, and then placed in a Harwell chamber and observed at a magnification of 400x under a microscope. The average spermatid levels in each sample were measured. The number of spermatids was calculated using Equation (1), and the value was divided by the weight of the decapsulated testicles per gram of testis to determine sperm production according to Equation (2).
Histological analysis
The histological analysis began by immersing the small testicular tissues in 10% formalin for 4 to 6 hours. Subsequently, the tissues were dehydrated using different concentrations of alcohol (ranging from 60% to 100%). The dehydrated tissues were then used to create paraffin tissue blocks. A microtome was employed to cut the tissues into thin slices measuring 5 mm in thickness. The staining procedure utilized the hematoxylin-eosin staining technique [25]. The ribbons containing the tissues were stretched out and fixed to clean, albumenized glass slides, which were placed on a Fisher slide warmer set at 60 °C. These glass slides were then incubated for approximately twelve hours to complete the stretching process and eliminate any residual bubbles. The 5 mm thick slices were viewed through light microscopy at magnifications of 40x and 100x. Each slide from both the control and treatment groups was examined and captured using a camera [26].
Statistical analysis
Statistical analysis of the data was performed using ANOVA in a general linear model, with a significance level set at p < 0.05. The Minitab 17 software was employed for the statistical analysis. Tukey's test was used for the comparison of means among multiple treatment groups, with a significance level of p < 0.05.
Results and Discussion
Characterization of TiO2-NPs
TiO2 nanoparticles were prepared using the Sol-Gel method and subjected to characterization through various techniques, including XRD and FT-IR. The X-Ray Diffraction (XRD) analysis revealed that the average size of the nanoparticles was approximately 25 nm, with a size range spanning from 25 nm to 55 nm. The XRD graph displayed distinctive peaks corresponding to anatase TiO2-NPs across different size ranges present in the samples.
The highest peak was observed at 25.00 nm, while other peaks ranged from 30 nm to 55.00 nm. The average crystalline size of NPs was 25 nm.
FT-IR spectroscopy is commonly employed to identify the major factors of absorption of frequency of two atoms. The graph depicting the spectra of TiO2-NPs clearly illustrates a significant difference in the spectra, notably the absence of C-H stretching at 3029.65 cm-1 and bending at 1622.72 cm-1 in the compound. This observation suggests that the heavier atoms within the compound have formed bonds absorbed at lower frequencies.
General observations
Throughout the entire duration of the experiment, all the rats were subjected to daily close observation for a week. Rats in both the control and TiO2-NPs-treated groups exhibited good health and displayed energetic movements. Their food and water intake were frequent and normal. Initially, the rats exposed to TiO2-NPs appeared normal and physically healthy. However, as the experiment progressed, these rats began to exhibit slower and more sluggish behavior. Remarkably, their food and water intake remained consistent with that of the control group. Specifically, during the first week of the experiment, the TiO2-NPs-treated rats were normal and active. However, as the duration of treatment increased, these rats became progressively slower and more sluggish compared to the control group. Some of them even developed eye infections. Among the animals treated with 50 mg/kg of TiO2, no significant deviations in health status or behavior were observed throughout the study. Conversely, rats treated with 90 and 130 mg/kg of TiO2, after seven days of exposure, displayed signs of irritability and reduced physical activity. Importantly, there were no reported fatalities in any of the treatment groups. Upon completion of the 28-day experimental period, the control group rats exhibited robust growth and good health. During dissection, it was noted that the control rats had soft skin, well-organized organs, and a rich blood supply, evident from their pinkish color. Fatty tissues were observed in the coelom at various locations. In contrast, the TiO2-NPs-treated rats exhibited dry skin, a shrunken body cavity, and reduced coelomic fluid. Their internal organs appeared to be devoid of fat. These observations collectively indicate the TiO2-NPs toxicity, particularly at the dose of 130 mg/kg.
Rats body weight and testosomatic index
The animals in the control and saline-treated groups exhibited normal behavior and showed a consistent increase in body weight over the course of the trial. However, it became evident that the animals administered medium and high doses of TiO2-NPs (130 mg/kg and 90 mg/kg, respectively) displayed aberrant behavior after two weeks of exposure. During the second and the third weeks of exposure, a significant decrease in body weight (135.70 ± 0.837c) was observed in the group (G2) treated with the moderate dose (90 mg/kg BW) of TiO2-NPs and the group (G3) treated with the high dose (130 mg/kg BW) of TiO2-NPs compared to the control (C), placebo (P), and the group (G1) treated with the small dose (50 mg/kg BW), (p < 0.05). However, no significant differences were observed in the first week of the experiment among the control and treated groups (p > 0.05) (Table 2). In addition, the administration of high dose of TiO2-NPs significantly reduced the testosomatic index (testes weight relative to body mass) compared to the control and placebo groups. This reduction was reflected in a testosomatic index of 0.25 ± 0.01B and 0.23 ± 0.02B (Table 3).
Testosterone level
Table 4 demonstrates that rats treated with different doses of titanium dioxide experienced a decrease in body weight and testosterone levels after 28 days of exposure. The most significant reduction was observed in the high-dose treatment group, namely G3 at 130 mg/kg BW of rats (Figure 1).
Table 2: Mean ± SE of weekly body weight (g) of the rats in the control group and the treatment groups
|
Group |
Control |
G1 |
G2 |
G3 |
G4 |
|
Initial weight |
105.90 ± 1.8 2A |
106.80 ± 2.59A |
105.80 ± 1.92A |
107.00 ± 1.58A |
106.00 ± 1.00A |
|
The 1st week |
121.10 ± 1.22A |
123.71 ± 1.2 0A |
122.40 ± 1.67A |
121.40 ± 1.52A |
120.60 ± 1.7 2A |
|
The 2nd week |
131.60 ± 1.67A |
130.20 ± 1.30A |
131.70 ± 1.79A |
131.60 ± 1.52A |
133.40 ± 1.52A |
|
The 3rd week |
143.20 ± 1.92A |
145.00 ± 1.22A |
141.40 ± 0.88 A |
138.40 ± 1.67B |
146.60 ± 1.4 2B |
|
The 4th week |
152.8 ± 3.56A |
152.2 ± 0.837A |
147.20 ± 1.46A |
135.70 ± 0.837c |
154.40 ± 1.52C |
Means bearing different letters in rows are substantially different (p < 0.05) compared to groups C and P
Table 3: Mean ± SE of the testosomatic index of the rats in the control group and the treatment groups
|
Group |
Parameter |
|
Testosomatic index |
Body Weight (g) |
|
C 2.01 ± 0.04A |
152.8 ± 3.35A |
|
G1 0.53 ± 0.16A |
152.2 ± 0.837B |
|
G2 0.25 ± 0.01 |
148.20 ± 1.47C |
|
G3 0.23 ± 0.02B |
135.70 ± 0.736D |
|
P 1.15 ± 0.04A |
154.40 ± 1.53A |
P 1.15 ± 0.04A; Means bearing different letters in rows are significantly different (p < 0.05)
Table 4: Mean ± SE of testosterone level of rats in the control group and the treatment groups
|
Group |
Parameter |
|
Testosomatic index |
Body Weight (g) |
|
C 3.5 ± 0.05A |
152.8 ± 3.35A |
|
G1 2.5 ± 0.06A |
152.2 ± 0.837B |
|
G2 2.0 ± 0.01B |
148.20 ± 1.47C |
|
G3 1.5 ± 0.01B |
135.70 ± 0.736D |
|
P 3.0 ± 0.04A |
154.40 ± 1.53A |
Means bearing different letters in rows have substantially different p-values (P < 0.05), specifically 152.2 ± 0.8B, 148.20 ± 1.47C, and 135.70±0.736D, respectively, compared to groups C and P
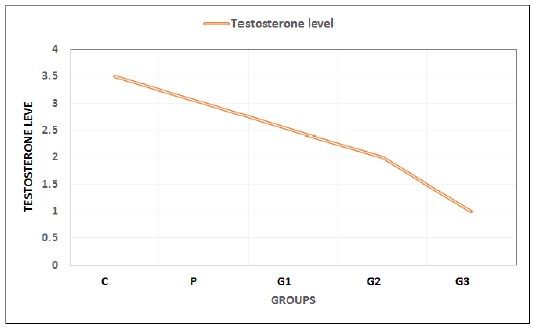
Figure 1: Testosterone levels of male Sprague Dawley rats in the control group and the TiO2-administered groups after 28 days of exposure. Values in this graph are expressed in Mean ± SD
production rate
In the present study, no changes were observed in sperm production (SP) rate in non-treated rats (p > 0.05) (Table 5). However, significant changes were noted in the case of G2 and G3 groups, the SP values of which substantially decreased with an increasing dose of titanium dioxide nanoparticles (p < 0.05) (Figure 2).
Hematological alterations
Table 6 presents the means of the blood parameters of the Sprague Dawley rats in the treatment and control groups during the 28-day study. Several hematological parameters exhibited dose-dependent changes in rats administered varying doses of TiO2-NPs. In G2 and G3, treated with medium (at 90 mg/kg BW) and high (130 mg/kg) doses of TiO2-NPs, all blood parameter concentrations were significantly higher (P < 0.05) compared to the control group (C), the placebo group (P), and G1 (treated with smaller doses, i.e. at 50 mg/kilogram BW). The highest mean values for all blood parameters were observed in G3 (treated with 130 mg/kg BW of TiO2-NPs) with values of 10.905 ± 0.453, 4.5921 ± 0.1430, 89.311 ± 0.445, 19.366 ± 0.233, 28.943 ± 0.537, 65.091 ± 0.593, 45.092 ± 0.853, and 1299.3 ± 7.84. Similarly, for additional blood parameters, the values in G3 were higher compared to the control groups (groups C and P).
Liver profile
Table 7 indicates the toxic effects of TiO2-NPs on the liver function of the male Sprague Dawley rats. It reveals low levels of alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and aspartate aminotransferase (AST) in the control group.
Table 5: Mean ± SE of sperm production (SP) rate of the rats in the control group and the treatment groups after 28 days of exposure
|
Group |
Sperm production rate 108/mL |
|
C |
40 ± 0.05A |
|
G1 |
25.5 ± 0.06A |
|
G2 |
15.5 ± 0.01B |
|
G3 |
5.5 ± 0.01c |
|
P |
35.5 ± 0.04A |
Means with different letters in rows are significantly different (p < 0.05)
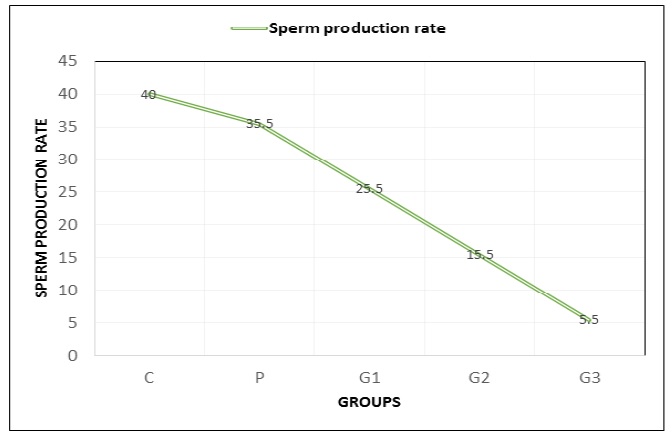
Figure 2: Sperm production rate of the male Sprague Dawley rats in the control group and the TiO2-NPs-treated groups after 28 days of exposure. Values in the graph are expressed as Mean ± SD
Table 6: Mean ± SE of hematological parameters of the Sprague Dawley rats in the control group and the treatment groups after 28 days of trial
|
Group |
Control |
G1 |
G2 |
G3 |
P |
|
WBC |
6.678 ± 0.1651 |
6.217 ± 0.0163 |
8.3261 ± 0.1486 |
10.903 ± 0.462 |
6.6681 ± 0.1640 |
|
RBC |
6.584 ± 0.2077 |
6.512 ± 0.255 |
5.9170 ± 0.0422 |
6.5921 ± 0.1430 |
6.5821 ± 0.2078 |
|
LYM% |
46.455 ± 0.932 |
46.457 ± 0.930 |
67.730 ± 0.805 |
89.311 ± 0.447 |
46.455 ± 0.932 |
|
MCH |
14.14 ± 0.181 |
14.12 ± 0.181 |
14.23 ± 0.170 |
19.375 ± 0.233 |
14.14 ± 0.170 |
|
MCHC |
17.167 ± 0.358 |
17.165 ± 0.369 |
20.595 ± 0.357 |
28.943 ± 0.537 |
17.167 ± 0.358 |
|
MCV |
42.535 ± 0.648 |
42.535 ± 0.648 |
54.401 ± 0.348 |
65.083 ± 0.594 |
42.535 ± 0.649 |
|
HCT |
22.223 ± 0.580 |
22.221 ± 0.582 |
39.339 ± 0.548 |
45.092 ± 0.853 |
22.222 ± 0.581 |
|
PLT |
493.01 ± 5.33 |
644.81 ± 15.43 |
875.21 ± 19.52 |
1299.3 ± 7.86 |
534.60 ± 15.77 |
|
GRA |
0.281 ± 0.294 |
0.281 ± 0.296 |
0.270 ± 0.296 |
0.17 ± 0.0895 |
0.281 ± 0.296 |
|
GRA% |
4.51 ± 3.42 |
4.53 ± 3.40 |
4.62 ± 3.31 |
5.57 ± 2.43 |
4.51 ± 3.42 |
|
HGB |
14.88 ± 0.198 |
13.619 ± 0.142 |
11.267 ± 0.132 |
14.967 ± 0.498 |
14.821 ± 0.526 |
|
RDW-SD |
14.255 ± 0.319 |
34.167 ± 0.232 |
36.021 ± 0.535 |
39.831 ± 0.0371 |
14.257 ± 0.483 |
|
RDW-CV |
13.717 ± 0.631 |
12.361 ± 0.220 |
13.377 ± 0.171 |
15.695 ± 0.162 |
13.587 ± 0.383 |
|
MPV |
6.491 ± 6.101 |
6.370 ± 0.227 |
7.401 ± 0.273 |
7.557 ± 0.342 |
6.201 ± 0.264 |
|
PDW |
14.03 ± 0.698 |
13.377 ± 0.714 |
7.6501 ± 0.2061 |
7.2541 ± 0.0476 |
14.207 ± 0.556 |
|
PCT |
0.50801 ± 0.0073 |
0.5961 ± 0.0638 |
0.7801 ± 0.0446 |
1.0201 ± 0.1303 |
0.5601 ± 0.0293 |
Means in columns are significantly different at P < 0.05, with values of 10.904 ± 0.463, 4.5920 ± 0.1431, 89.310 ± 0.446, 19.376 ± 0.232, 28.942 ± 0.538, 65.092 ± 0.592, 45.092 ± 0.853, and 1299.2 ± 7.85, 0.16 ± 0.0894, 5.58 ± 2.42, 8.966 ± 0.499, 39.830 ± 0.0381, 15.694 ± 0.163, 7.558 ± 0.341, 7.2540 ± 0.0477, and 1.0201 ± 0.1303, respectively, compared to groups C and P
profile
Table 7 indicates the toxic effects of TiO2-NPs on the liver function of the male Sprague Dawley rats. It reveals low levels of alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and aspartate aminotransferase (AST) in the control group. In contrast, rats treated with different concentrations of TiO2-NPs showed a substantial increase (p < 0.05) in ALT, AST, ALP, and LDH levels in a dose-dependent manner.
Kidney profile
Table 8 presents the toxic effects of TiO2NPs on the kidney function of the male Sprague Dawley rats. Various kidney parameters, including creatinine (CRE), uric acid (UA), urea, total bilirubin (TBIL), and blood urea nitrogen (BUN), were observed in the control group and the treatment groups. The rats treated with different doses of TiO2-NPs exhibited a significant decrease in CRE, UA, and BUN (p < 0.05), while there was a significant increase in urea and TBIL levels in a dose-dependent manner.
Table 7: Mean ± SE of liver profile (IU/I) of the male sprague dawley rats in the control group and the treatment groups after 28 days of exposure
|
Group |
|
Parameter |
|
|
|
|
ALT |
AST |
LDH |
ALP |
|
C |
13.42 ± 0.13D |
44.62 ± 1.29D |
1336.89 ± 5.31D |
53.88 ± 0.68D |
|
S |
13.58 ± 1.80D |
44.38 ± 1.47D |
1336.22 ± 4.71D |
53.76 ± 1.24D |
|
G2 |
132.02 ± 2.55B |
52.66 ± 1.32 B |
2127 ± 21.75B |
57.36 ± 1.28B |
|
G3 |
142.63 ± 2.10A |
55.34 ± 1.32A |
2635 ± 29.2 6A |
61.22 ± 1.65A |
Means that do not share the same letters in columns are significantly different (p < 0.05)
Table 8: Mean ± SF of kidney profile (mg/dl) of the male sprague dawley rats in the control group and the treatment groups after 28 days of exposure
|
Group |
CRE |
Parameter (UA) |
Urea |
TBIL |
BUN |
|
C |
1.11 ± .0.11A |
4.75 ± 0.011A |
48.12 ± 2.12D |
0.39 ± 0.24D |
20.71 ± 0.2 3A |
|
S |
1.12 ± 0.01A |
4.71 ± 0.054A |
47.62 ± 1.81D |
0.41 ± 0.13D |
20.72 ± 0.27A |
|
G1 |
1.06 ± 0.12A |
4.29 ± 0.047B |
52.43 ± 2.27C |
0.51 ± 0.22C |
21.70 ± 0.31A |
|
G2 |
0.81 ± 0.01B |
3.70 ± 0.115C |
58.02 ± 2.12B |
0.80 ± 0.05B |
16.01 ± 0.0 3B |
|
G3 |
0.60 ± 0.01B |
3.50 ± 0.012D |
70.03 ± 2.9 1A |
0.97 ± 0.08 A |
15.13 ± 0.2 3B |
Means that do not share the same letters in the column are significantly different (p < 0.05)
Histological alterations in testes
The testes of the control group rats were oval-shaped, covered with fatty tissues, and exhibited a healthy pink color, indicative of a rich blood supply. The normal and healthy testes were easily detached from the tissues, and the outer layer, tunica albuginea, could be removed easily from the parenchymatous tissues. However, in the TiO2-treated rats, the separation of reproductive organs was not as straightforward. While the testes in the control group were normal in volume, they appeared pale in color due to reduced blood supply. Fat content around the testes was less, and the tunica was firmly attached to the tissues. The testes were pink in color due to a normal blood supply, and the outer layer could be easily detached from the internal tissues. In comparison to the control group, these testes had a thinner layer of fat and appeared yellowish due to diminished blood supply. At a dose of 50 mg/kg BW of TiO2-NPs exposure in G1, the normal structure of the testes (control/P group) could be observed under a microscope (Figure 3). This structure included a seminiferous tubule lumen (L) filled with sperm, a well-defined basement membrane (Bm), spermatogonia (Sg), spermatocytes (ST), primary spermatocytes (Ps), and spermatozoa (Sz). However, histological alterations were observed in the groups that received 50 mg/kg BW of TiO2-NPs treatment. The seminiferous tubules exhibited mineralization both inside and outside of them, vacuolation (Va), an inflammatory response with the production of vacuoles (Vc), and disruptions to the basement membrane (Bm) (MIN) were all noticeable histological alterations. The testicular tissues of the rats in G2, exposed to a dose of 90 mg/kg BW, exhibited necrosis (cell death) or loss of cells, loss of the spermatogenic series (LSC), vacuolation, a band of germ cell absence (SGC), and mineralization (MIN) (Figure 3). Serious histological damage, including sloughing (Sl), necrosis (N), and large lumens (L) devoid of sperm cells, were observed in G3 at a dose of 130 mg/kg BW. Cells were entirely separated from Sertoli cells and detached from the basement membrane's somniferous tubules (SC) (Figure 3). This study revealed negative histological alterations in the testicular architecture with greater exposure to TiO2-NPs and fewer alterations at lower doses. The testes of the rats in the control group were circular, with elongated seminiferous tubules of varying diameters, densely packed with germ cells.
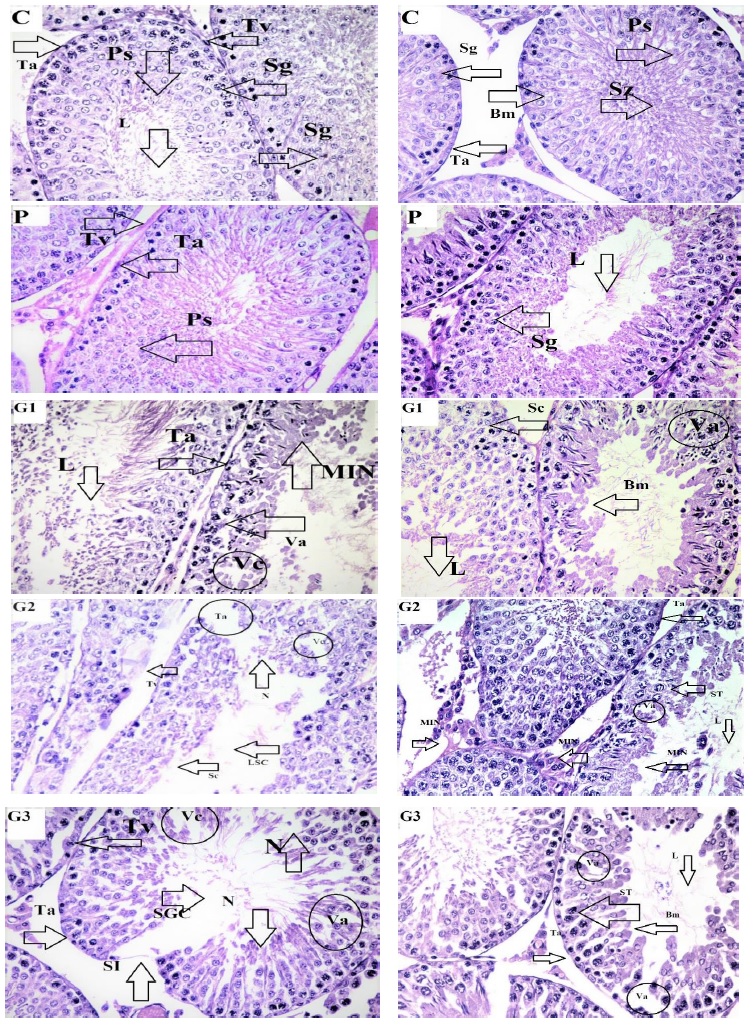
Figure 3. Microscopic image of hematoxylin and eosin-stained testicular tissues of male rats treated with TIO2: The testicular tissue sections of Groups C and P exhibited normal histology. In G1, treated with a low dose (50 mg/kg BW), mild histological alterations were observed, including mineralization (MIN) inside and outside the membrane, wide lumen, and vacuolation in cells (Va). In G1, treated with a medium dose (90 mg/kg BW), histological alterations were evident, with a wide lumen, damaged outer membrane of tissues (Ta), and mineralization (MIN). In G3, treated with a high dose (130 mg/kg BW), severe tissue damage was observed, characterized by cell necrosis (N), sloughing (Sl), and vacuolation (Vc)
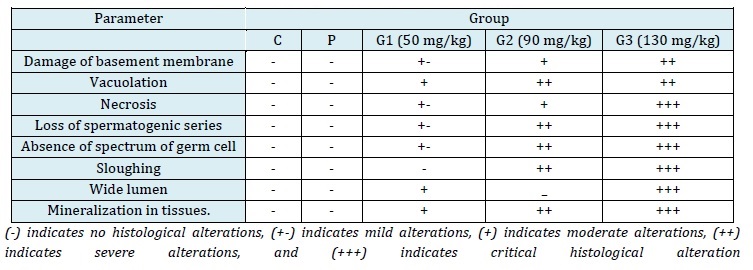
Table 9: Comparison of histological alterations in the male Sprague Dawley rats in the control group and the treatment groups after 28 days of TIO2 exposure
This study was designed to assess the toxic effects of TiO2-NPs on the gonad histology, testosterone levels, testosomatic index, sperm production rate, and blood parameters (gonadal functions and CBC test) of male Sprague Dawley rats. The rats were exposed to TiO2-NPs at doses of 50 mg/kg, 90 mg/kg, and 130 mg/kg for 28 days. Throughout the study, no mortality was observed in any of the groups, but alterations in behavior, such as an increased tendency to cannibalize other rats and reduced appetite, were noted in the G3 (130 milligram/kg) and G2 (90 milligram/kg) groups, while the G1 (50 milligram/kg) group exhibited normal behavior. These behavioral alterations in rats align with the findings of Amara et al. (2015), who investigated emotional and pathophysiological alterations in rats induced by certain drugs [27]. TiO2 nanoparticles continue to pose risks to both humans and the environment due to their presence in the environment and the food chain [28]. In this present study, an increase in the TiO2-NPs dose resulted in a decrease in the body weight of the rats, along with a reduction in the testosomatic index. These findings are consistent with those of Sharma et al. (2014), who examined the effects of intravenously injected TiO2-NPs on rats [29]. Similarly, Amara et al. (2015) reported similar results when studying the reproductive system, biochemical alterations, and emotional behavior in rats [27].
Hu et al. (2020) also observed a reduction in body weight in mice following exposure to Ni-NPs, which is in line with the weight loss observed in the rats exposed to TiO2-NPs in our study [30]. After exposure to Ni-NPs and silver nanoparticles, a decrease in body weight was also observed in rats.
The hematological analysis in our study revealed significant improvements in all blood parameters. These findings are consistent with the observations made by Xu et al. (2013) and Grissa et al. (2016), who assessed the acute toxicity, induced by TiO2 NPs, and similarly reported an increase in all blood parameters [31, 32]. Wang et al. (2022), in their study on how TiO2-NPs affect bone regeneration and healing, also noted a similar elevation in blood parameters [33]. According to Shakeel et al. (2016), an increase in mean corpuscular volume (MCV) may result from disruptions in mitotic division, while an increase in white blood cells (WBCs) could be attributed to a stress response that modulates the immune system [34]. Similar alterations in hematological parameters were documented by Younes et al. (2015) in their assessment of subacute toxicity induced by TiO2-NPs [35].
Hong et al. (2016) observed that TiO2 nanoparticles have an impact on spermatogenesis [36]. Rats exposed to TiO2 NPs for an extended period of sixty consecutive days exhibited alterations in daily caloric and fluid intake, overall body weight, relative testicular weight, sperm counts, and motility. They also experienced an increase in the percentage of abnormal sperm, sperm lesions in the cauda epididymis, and structural changes in the testis and epididymis.
The findings of our study align with the work of Khorsandi et al. (2017), Li et al. (2018), and Negahdary et al. (2015), who observed that TiO2 and Mn2O3 nanoparticles induced a significant decrease in testosterone levels in healthy male rats with increasing NPs exposure [37-39]. These observations suggested that increased exposure to Ni-NPs led to testicular damage and impaired spermatogenesis in the testes, resulting in decreased blood testosterone levels [40].
Orazizadeh et al. (2014) reported that after 35 days of oral ingestion of 300 mg/kg of TDN (Titanium Dioxide Nanoparticles), there were no appreciable changes in the body weight of mice [41]. In contrast, the present study found that oral administration of TDN resulted in a significant decrease in the relative weights of the testes, epididymis, and seminal vesicles compared to the control group at the first euthanasia. However, at the second euthanasia, only a non-significant decrease in the relative weights of the prostate and seminal vesicles was observed compared to the control group.
This study aligns with the study by Meena et al. (2014), who administered anatase TiO2-NPs to adult male Wistar rats once a week for four consecutive weeks and observed reduced sperm numbers at a cumulative dose of 200 mg/kg [42]. In this study, the cumulative dose was 17 mg/kg, significantly lower than the effective dose.
Hu et al. (2020) investigated histopathological abnormalities, including sloughing in testes, damage to spermatogonia (Sg), primary spermatids (Ps), spermatozoa (Sz), and the seminiferous tubule's lumen (L), as well as changes to the basement membrane (Bm), formation of vacuoles in necrotic tissues (Vc), and mineralization both within and outside the tubule (MIN) [30]. A spectrum of germ cell absence (SGC), separation of Sertoli cells, and loss of spermatogenic series (LSC) were also observed. These findings are similar to those in Sprague Dawley rats treated with titanium dioxide nanoparticles, which led to increased reactive oxygen species (ROS) generation and similar histological results.
The renal function test in our study revealed a dose-dependent significant increase in ALT, AST, and ALP enzymes, consistent with the findings of Abbasi-Oshaghi et al. (2019) [43]. They observed that TiO2-NPs induced oxidative stress and necrosis in the kidneys and intestines of rats. Vasantharaja et al. (2015) and Amara et al. (2013) provided evidence that the increase in these enzymes was due to the rupture of renal membranes and the leakage of enzymes into the bloodstream [27].
Conclusion
In conclusion, this study demonstrated that male Sprague Dawley rats exposed to TiO2-NPs experienced dose-dependent blood and gonadal toxicity. The exposure to TiO2-NPs reduced the rats' physical activity levels and led to significant hematological and histological alterations. Overall, the findings of this study highlight the harmful effects of TiO2-NPs on gonadal function and blood parameters in rats.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
ORCID
Adeel Khalid
https://orcid.org/0000-0001-5064-4670
Asma Asghar
https://orcid.org/0009-0004-7690-293X
M. Danish
https://orcid.org/0000-0001-6361-4016
Ayesha Ijaz
https://orcid.org/0009-0004-1141-4628
Ammara Zahid
https://orcid.org/0009-0000-5437-8373
Arif Nur Muhammad Ansori
https://orcid.org/0000-0002-1279-3904
Ahmad Affan Ali Murtadlo
https://orcid.org/0000-0002-7942-875X
Rahadian Zainul
https://orcid.org/0000-0002-3740-3597
HOW TO CITE THIS ARTICLE
Adeel Khalid, Asma Asghar, M. Danish, Ayesha Ijaz, Ammara Zahid, Arif Nur Muhammad Ansori, Ahmad Affan Ali Murtadlo, Rahadian Zainul, Effectivity and Safety after Intravitreal Triamcinolone Acetonide and Bevacizumab Titanium dioxide (TiO2) nanoparticles induced gonadal toxicity in exposed male Sprague-Dawley rats. J. Med. Chem. Sci., 2024, 7(1) 150-165.