Document Type : Original Article
Authors
- Riveliño Ramon-Curay 1
- Darwin Robalino Salas 2
- Nelly Tacle García 2
- Jenny Martínez Moreira 1
- Favian Bayas-Morejón 1
1 Universidad Estatal de Bolívar, Facultad de Ciencias Agropecuarias, Recursos Naturales y del Ambiente. CP: 020150, Guaranda, Ecuador
2 Centro Veterinario Animals Distrivet, CP: 020302, San José de Chimbo, Ecuador
Abstract
Staphylococcus aureus is a Gram-positive pathogen that is commonly associated with bovine mastitis, which is an infectious condition affecting the udder of cows. The objective of the present study is to evaluate the antimicrobial efficacy of methicillin, amoxicillin, and ampicillin for detecting methicillin-resistant Staphylococcus aureus (MRSA) strains isolated from cases of bovine mastitis. Methodologically, it was established to carry out the antimicrobial activity using the plate disc diffusion method (Kirby-Bauer) with the use of amoxicillin and ampicillin at 2 µg and 10 µg, also following the recommendations of the Clinical and Laboratory Standards Institute. To detect resistant methicillin strains, oxacillin was used at 1 µg, and cefoxitin at 30 µg. The study was conducted against 50 strains of the bacteria under investigation, which were isolated and identified. The applied treatments demonstrated a highly significant effect (p<0.05). In addition, a resistance of 50% and 60% to concentrations of 2 µg of amoxicillin and ampicillin, respectively, was observed. Furthermore, a resistance of 60% and 68% to 10µg of amoxicillin and ampicillin, respectively, was observed, indicating that Staphylococcus aureus is developing mechanisms that confer antimicrobial resistance. To further investigate this, the antimicrobial activity was conducted using 1 µg oxacillin discs and 30 µg cefoxitin, revealing that 36% and 32% of the isolates were resistant to these drugs, respectively. Phenotypically, 32% (n=16) of the isolates were identified as methicillin-resistant Staphylococcus aureus (MRSA), demonstrating resistance to all beta-lactams tested.
Graphical Abstract
Keywords
Introduction
Worldwide economic losses from mastitis in dairy systems represent approximately 26% of total costs. Mastitis is the most prevalent infectious disease in adult dairy cattle. Several species of bacteria, fungi, mycoplasmas, and algae have been isolated from the disease or have been shown to reproduce it experimentally [1].
The inflammation, characteristic of mastitis may be undetectable without the use of diagnostic tests applied to the milk or discharge. This condition is known as subclinical mastitis. It is important to note that mastitis is a consequence of intramammary infection caused by one or more microorganisms from various genera. These microorganisms find a nutritious substrate in the mammary gland, which promotes their growth and multiplication, contributing to the mastitis development [2].
Among these microorganisms, the bacterium Staphylococcus aureus stands out and can cause a wide range of diseases, ranging from relatively minor skin infections to serious and life-threatening infections within dairy herds. These include conditions such as endocarditis, omphalophlebitis in calves, pneumonia, sepsis, multi-organ abscesses, and severe clinical mastitis. In addition, its impact is reinforced by the development of resistance to antibiotics, especially methicillin-resistant Staphylococcus aureus (MRSA) which is resistant to practically all β-lactam antibiotics [3].
Within the dynamics of the infection, the mammary glands can be classified into one of three categories: uninfected, subclinically infected, or clinically infected, the type of pathogens also influences this classification [2].
Coliform infections tend to become clinical rapidly, whereas Staphylococcus aureus infections often persist as subclinical infections for weeks or months. Bacteriologic cure rates range from 90% for most coliform infections to 25% with Staphylococcus aureus in older cows [4].
It should be noted that the infected teat is a direct route of transmission, which occurs during the milking process. Most infections are subclinical and lead to an increase in cell count. Hygienic and sanitary measures are the best control measures [5].
Contagious bacteria include Staphylococcus aureus, coagulase-negative Staphs, Streptococcus agalactiae, and Strep. Dysgalactiae, with a herd somatic cell count greater than 200,000 mL [6].
It has been shown that it is possible to eradicate Staphylococcus aureus from individual cases by implementing hygienic measures during milking and by discarding chronically infected cows. However, the main limitation for achieving more effective control of Staphylococcus aureus remains its poor response to antibiotic therapy [7].
Experimentally, the in vitro demonstration of the sensitivity of Staphylococcus aureus to an antibiotic is not a guarantee of therapeutic success [8].
The ability of bacteria to survive within immune cells or polymorphonuclear cells, such as macrophages and epithelial cells, confers protection against the action of antibiotics. This is a starting point to believe that it can significantly contribute to their resistance to therapy. Furthermore, the pathological changes, particularly granulomas and fibrosis, induced in Staphylococcus aureus, render chronically infected cows essentially incurable. Even acute gangrenous infections can result in uncontrolled growth of the microorganism, producing large amounts of a toxin [9, 10].
The antibiotics most commonly used to control mastitis are those of the beta-lactam group. The mechanism of action of this group of antibiotics is the inhibition of cell wall synthesis. However, the main resistance mechanism is the beta-lactamases production by microorganisms causing clinical infections [11, 12].
Beta-lactams are partial bactericidal agents since they only act during the cell growth phase, and their efficacy is time-dependent, occurring based on the minimum inhibitory concentration. However, their rapid bactericidal capacity can cause the release of endotoxins, leading to a heightened inflammatory response. It should be noted that more than 90% of Staphylococcus aureus strains are resistant to penicillins [13].
Beta-lactam classification
Penicillins
Natural penicillins, such as Penicillin G, semisynthetic penicillins, penicillinase-resistant penicillins, beta-lactamase inhibitors, cephalosporins (including the first, the second, and the fourth generation cephalosporins), monobactams, carbapenems, and methicillin are all important classes of antibiotics used in the treatment of various bacterial infections [13-17]. With everything previously described, the objective of this study was to analyze the antimicrobial effectiveness of methicillin, amoxicillin, and ampicillin against methicillin-resistant Staphylococcus aureus (MRSA) strains isolated from bovine mastitis.
Materials and Methods
The present investigation was conducted in the General Laboratory of the Faculty of Agricultural Sciences, Natural Resources, and the Environment at the State University of Bolívar. A completely randomized design (CRD) was used for factor analysis, with the following description of the treatments (Table 1).
Obtaining the bacterial strains
The present investigation was conducted using strains of Staphylococcus aureus that were previously isolated from 50 cows positive for mastitis out of a total of 136 cows analyzed from the agricultural cooperative "La Colina" in San Miguel de los Bancos, Pichincha Province.
In addition, 20 cows positive for the disease out of 60 cows analyzed from the areas of La Candelaria and Releche in the Penipe, province of Chimborazo, were included. These bacterial isolates were preserved in MR-vP broth with 10% glicerol, and then recultured for the purpose of this study (Table 2).

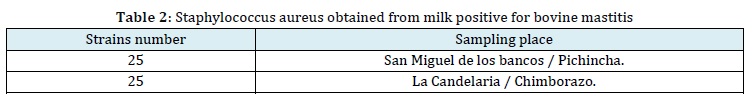
Staphylococcus aureus reactivation
Fifty strains of Staphylococcus aureus were selected from milk samples that tested positive for bovine mastitis. For the conditioning process, the strains were removed from the freezer and allowed to rest at refrigeration temperature, followed by room temperature for 1 hour, and then 1-5 µL of each strain were taken from the cryovial and inoculated into MR-vP broth. The inoculated broth was incubated at 37 °C for 24 hours. After obtaining the bacterial strain, it was inoculated onto salt mannitol agar plate, in duplicate, to obtain a pure and homogeneous culture. The inoculation was performed by spreading the strain over the entire surface of the agar plate using a swab. The plates were then incubated for 24 hours at 37 °C.
Identification of staphylococcus aureus strains
The identification process involved macroscopic and microscopic description of the phenotypic characteristics, colony morphology, mannitol fermentation, and metabolic pathways of Staphylococcus aureus. As a part of the methodology, the following tests were established, and the characterization parameters are presented in Table 3.
The growth morphology of Staphylococcus aureus is evidenced as smooth, shiny, and convex colonies, These colonies typically display an orange or porcelain white end pigment (Beta-carotenoid) and can even exhibit a golden color in the specific culture medium. Staphylococcus aureus is a Gram-positive bacterium that can be observed as single cells, pairs, tetrads, or larger groupings arranged in clusters resembling grapes [18]. Mannitol fermentation is a characteristic of Staphylococcus aureus. When grown in a medium with a high salt concentration and mannitol as the carbohydrate source, Staphylococcus aureus has the ability to ferment mannitol, producing acids.
This fermentation process leads to a decrease in pH, resulting in a color change of the culture medium from pink to pale yellow.
Catalase test, the catalase enzyme breaks down hydrogen peroxide into water and oxygen [16].
The Coagulase test: Staphylococcus aureus has two types of coagulase, one is endo-coagulase or bound coagulase (clumping factor), which causes the formation of clots or lumps in the blood plasma. It also has an exocoagulase or free coagulase that produces a fibrin clot [19].
This test was performed as follows: Initially, 10 mL of blood was obtained in Vacutainer tubes. The cellular component, leukocyte platelet, and plasma were separated by centrifugation at 3000 rpm for a period of 5 minutes. 1 mL of plasma was then transferred to Eppendorf tubes. Subsequently, a bacterial inoculum was added to the plasma. After incubation for 4 to 6 hours, clot formation can be observed.
Oxidase test
It is a colorimetric test that allows determining the presence of oxidase enzyme, which reacts in the presence of cytochrome oxidase present in the reactive strips. The test was carried out as follows: A bacterial colony was taken and placed on the test strip to observe the reaction. It is considered positive when a blue or purple coloration is observed on the test strip.
Antimicrobial activity
The analysis of antimicrobial activity (disc diffusion method) was carried out as follows: A bacterial loop was taken from a previously incubated culture, this was suspended in 5 mL of sterile distilled water. The McFarland scale was used to adjust the suspension to a turbidity equivalent to 0.5. With the help of a sterile swab, the bacterial suspension was evenly spread over the entire surface of the agar plate.

Finally, cellulose filter paper discs impregnated with the antibiotics under study were placed on the agar surface. The plates were then sealed and incubated at 37 °C for 24 hours.
After incubation, the diameter of the inhibition zones was measured using a vernier caliper. The measurement was adjusted by subtracting 6 mm (the diameter of the disc) from the recorded value, and the result was recorded on the data sheet for further analysis. Within the methodological process, the antimicrobial susceptibility of ampicillin and amoxicillin at the concentration of 2 µg was established according to the EUCAST [20] recommendations.
Prevalence analysis
To estimate the occurrence of previously obtained methicillin-resistant Staphylococcus aureus, the resistance and susceptibility data were used to calculate the prevalence rate (PT). The following equation model was employed to determine the result as a percentage [21]:
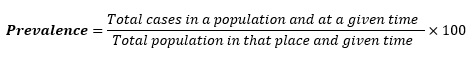
This equation allows us to calculate the prevalence rate of methicillin-resistant Staphylococcus aureus based on the obtained data.
Results and Discussion
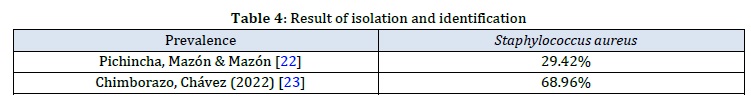
The Staphylococcus aureus isolates were taken from the strain collection of the general laboratory of the Faculty of Agricultural Sciences and Natural Resources of the State University of Bolivar, which were previously obtained from two provinces of Ecuador (Table 4).
After performing bacterial identification tests such as mannitol fermentation, growth in a saline medium, catalase reaction (+), oxidase reaction (-), coagulation of blood plasma, and Gram staining (Gram-positive cocci arranged in clusters resembling bunches of grapes), it was determined that 100% of the isolates were confirmed as coagulase-positive Staphylococcus aureus.
According to Falla et al. [24], in their investigation of the prevalence of Staphylococcus aureus that causes bovine mastitis, they obtained 129 isolates out of 150 sampled cows, resulting in a prevalence of 80%. This finding differs from the results of previous investigations from which the strains were obtained, as they reported a lower proportion of Staphylococcus aureus as the cause of bovine mastitis.
Comparison of means of the treatments of 2 µg of Ampicillin and Amoxicillin versus Staphylococcus aureus
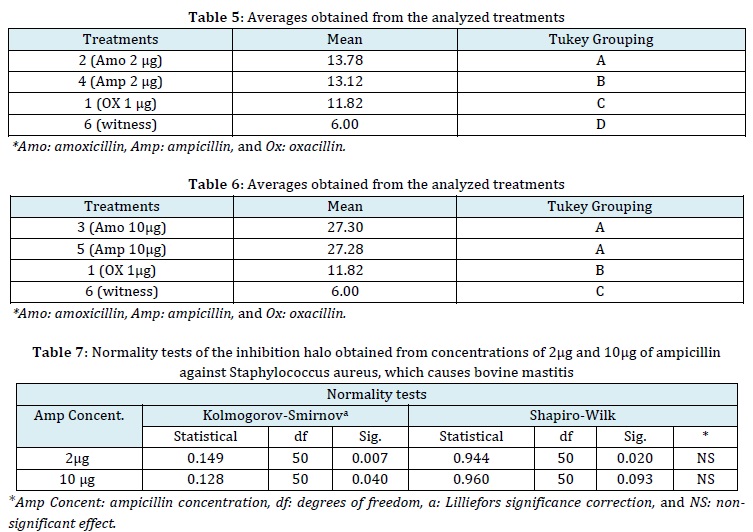
In the means comparison using the 5% Tukey test (Table 5), it was observed that T2 (2 µg of amoxicillin) had the highest average zone of inhibition of Staphylococcus aureus growth, significantly surpassing T4 (2 µg of ampicillin). There were statistic differences among the treatments according to the grouping of this comparative test. Treatment T1 (1 µg of oxacillin), which served as the positive control treatment, also showed a significant difference compared to the negative control (Disc impregnated with NaCl 0.9%), as no inhibition of bacterial growth was observed with the latter. These findings suggest that the treatment averages are statistically distinct from each other. Damasceno [25], in his research, isolated a total of 79 strains of Staphylococcus aureus, which accounted for 36.9% of the total isolates. The study demonstrated that among these isolates, 63.3% (n=50) were sensitive to 1 µg oxacillin, while 36.7% (n=29) showed resistance to this antibiotic. In the case of 10 µg amoxicillin, 65.8% (n=52) of the isolates were found to be sensitive, while 34.2% (n=27) exhibited resistance. Nevertheless, the EUCAST [20] recommendations for antimicrobial susceptibility tests consider isolates sensitive to ampicillin and amoxicillin at 2 µg when they exhibit inhibition zones ≥ 15 mm in the antibiogram. Therefore, the findings of the present investigation are in disagreement with these recommendations, as the 50 strains of Staphylococcus aureus exhibited inhibition zones with average measurements of 13.78 mm and 13.12 mm for amoxicillin and ampicillin, respectively. However, the data distribution reveals the presence of isolates with larger inhibition zone measurements than the established averages. This suggests the existence of sensitive bacteria with inhibition zones greater than the thresholds established by the above-mentioned regulatory body.
Comparison of means of the concentration of 10 µg of amoxicillin and ampicillin versus Staphylococcus aureus
In the comparison of means determined by the 5% Tukey test (Table 6), it was observed that T3 (10 µg of amoxicillin) and T5 (10µg of ampicillin) did not express significant statistical differences, They exhibited similar inhibition zones. T1 (1 µg of oxacillin), which served as the positive control treatment, showed significant differences from the negative control (disc impregnated with 0.9% NaCl), as no inhibition was observed in the latter. Khan et al. [26] in their investigation on antimicrobial susceptibility of beta-lactamase-producing Staphylococcus aureus isolated from milk positive for bovine mastitis reported that 53.3% (n=8/15) of the isolates showed phenotypic resistance to 10µg ampicillin, with halos ≤28 mm [27]. Mesquita et al. [28] obtained an average diameter of 18.53 mm for the inhibition zone of Staphylococcus aureus (ATCC 25923) against 10 µg amoxicillin. However, this finding disagrees with the cited investigations. It is important to note that the measurement of inhibition zones can vary due to various factors, including the conditions of antimicrobial handling in bovine mastitis, the underlying factors contributing to intramammary colonization, and the potential cross-transmission of nosocomial pathogens from humans to animals, such as MRSA.
Analysis of the antimicrobial activity of ampicillin against Staphylococcus aureus, which causes bovine mastitis
According to the normality test performed on the concentrations of 2 µg and 10 µg of ampicillin on 50 strains of Staphylococcus aureus (Table 7), the Shapiro-Wilk test indicates that the inhibition zones follow a normal distribution. This suggests that the data is normally distributed, and there is no significant effect observed between repeated measurements.
Antimicrobial susceptibility of ampicillin against Staphylococcus aureus causes bovine mastitis

According to CLSI [27], the following cutoff points are considered for all penicillins against Staphylococcus aureus: inhibition zones ≥ 29 mm are classified as sensitive, while inhibition zones ≤ 28 mm are classified as resistant. Similarly, EUCAST [20] recommends the use of 2 µg of ampicillin in disk diffusion method for in vitro studies, where inhibition zones ≤ 13 mm are considered resistant and inhibition zones ≥ 15 mm are considered sensitive (Table 8).
Antimicrobial susceptibility of ampicillin against Staphylococcus aureus, which causes bovine mastitis
The antimicrobial susceptibility testing of Staphylococcus aureus with ampicillin at a concentration of 10 µg revealed that only 32% of the isolated strains (n=16) were classified as sensitive, while the remaining 68% (n=34) were classified as resistant based on the recommendations provided by CLSI [27]. Similarly, when analyzing the interaction of bacterial agent with ampicillin at a concentration of 2 µg, it was observed that 30% of the total isolates (n=15) exhibited resistance, while 60% (n=30) were classified as sensitive to this concentration. In addition, it was noted that 10% (n=5) showed intermediate resistance.
Jiménez et al. [9] in their investigation of resistance profiles by Staphylococcus aureus against 10 µg ampicillin, they obtained 81.5% sensitivity and 18.5% resistance, Sánchez et al. [29] of a total of 53 isolates, 60.3% (n=32) presented resistance to 10 µg ampicillin, this interaction being one of those with the highest resistance rates, Guzmán et al. [8] of 32 isolates observed that 86.6% presented resistance to 10 µg ampicillin, observing that when molecularly analyzing mentioned isolates, 100% presented the blaZ gene and 36.6% were positive for the mecA gene, and it is strongly linked to the phenotypic and genotypic presentation of resistance to penicillins and beta-lactams by the bacteria under study since it gives it the ability to generate beta-lactamases that inhibit the mechanism of action of beta-lactam drugs.
Analysis of the antimicrobial activity of amoxicillin against Staphylococcus aureus, which causes bovine mastitis
According to the normality test of the concentrations of 2 µg and 10 µg of amoxicillin against the strains of Staphylococcus aureus that cause bovine mastitis, the Shapiro-Wilk test indicates that the inhibition halos follow a normal distribution. Furthermore, the repetitions under study show no significant effect (p>0.05).
Antimicrobial susceptibility of amoxicillin against Staphylococcus aureus, which causes bovine mastitis
The CLSI [27] considers such cutoff points for all penicillins against Staphylococcus aureus as sensitive inhibition halos ≥ 29 mm and resistant inhibition halos ≤ 28 mm, as well as the EUCAST, [20] recommends the application of 2 µg of amoxicillin using the disk diffusion method for in vitro studies.
In this method, inhibition halos of ≤ 13 mm are considered resistant, while inhibition halos of ≥ 15 mm are considered sensitive.
Antimicrobial susceptibility of amoxicillin against Staphylococcus aureus causes bovine mastitis.
The antimicrobial susceptibility of Staphylococcus aureus in the interaction with amoxicillin at a concentration of 10 µg showed that only 40% of the isolated strains (n=20) were sensitive. However, the remaining 60% (n=30) were resistant according to the recommendations established by the CLSI [27].
When analyzing the interaction of bacterial agent under study with amoxicillin at a concentration of 2 µg, it was observed that, out of the total isolates analyzed, 32% (n=16) were resistant.
In addition, 50% (n=25) were manifested as resistant to this concentration. Furthermore, it was observed that 18% (n=9) of the isolates presented intermediate resistance.
These interpretative criteria were referenced to the provisions of the established guidelines. However, in the work carried out by Quispe et al. [30], where they isolated the bovine mastitis pathogen, 100% of the isolates were found to be sensitive to amoxicillin.
Analysis of the antimicrobial activity of oxacillin against Staphylococcus aureus causes bovine mastitis
According to the Shapiro-Wilk test, performed on 50 strains of Staphylococcus aureus (degrees of freedom) against 1 µg of oxacillin (Table 9 and 10), the distribution of the inhibition halo measurements was found to be non-normal. The test revealed a highly significant effect (p<0.05), indicating that the experimental units did not exhibit similar behavior in response to the inhibitory effect of the studied drug.
Antimicrobial susceptibility of oxacillin against Staphylococcus aureus causes bovine mastitis
The results obtained from the diameter of the inhibition halo were compared with the cutoff points used in the research by Farzaneh et al. [31] for Staphylococcus aureus, where the cut-off points for S. aureus is Sensitive 13 mm in halo diameter, intermediate 11 to 12 mm, and resistant 10 mm.
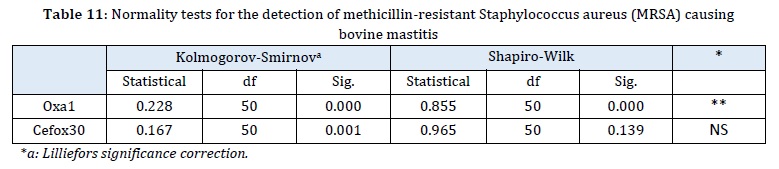
Table 11 indicates the susceptibility of Staphylococcus aureus to 1 µg of oxacillin, revealing that over 35% (n=18) of the isolates exhibited resistance to the drug. According to Gómez et al. [32], the emergence of oxacillin-resistant Staphylococcus aureus was observed in 1960. However, resistance is conferred by a modified penicillin-binding protein (PBP2a). This particular strain is recognized as a nosocomial pathogen primarily found in hospital settings and predominantly affecting humans. Hryniewicz and Garbaz [33] further explained that within oxacillin resistance, both homogeneous and heterogeneous phenotypic resistance can be observed. This resistance may be expressed by the entire bacterial population or only a portion, leading to the classification of Staphylococcus aureus as Bordeline (BROSA).
Prevalence of methicillin-resistant Staphylococcus aureus (MRSA)
For this analysis, the plate-disc diffusion method was applied with 1 µg oxacillin discs and 30 µg cefoxitin. The distribution of inhibition halos was then examined for the 50 strains studied in relation to these drugs.
According to the Shapiro-Wilk test, it was found that the distribution of inhibition halo measurements with the use of 1 µg oxacillin was heterogeneous among the 50 strains studied. This indicates that the isolates varied in their response to the inhibitory effect of the drug.
On the other hand, the distribution of inhibition halo measurements with the use of 30µg cefoxitin discs was observed to be homogeneous, indicating that the 50 isolates exhibited a similar response to the inhibitory effect of this drug. The cutoff points established for cefoxitin (30µg) against Staphylococcus aureus are: 22 mm of susceptible halo and 21 are considered resistant.
When analyzing the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) using the phenotypic analysis of resistance to 1 µg oxacillin and 30 µg cefoxitin, the CLSI parameters were used as a reference.
The mecA gene, which is associated with resistance to the mechanism of action of beta-lactam antibiotics, was considered in this analysis.
In a total of 50 strains of Staphylococcus aureus, it was found that 36% of the isolates were resistant to 1 µg oxacillin, 40% showed intermediate resistance, and the remaining 24% was sensitive to the drug.
In the case of cefoxitin with a concentration of 30 µg, 32% of the isolates were resistant, while 68% were sensitive to the drug.
Tesfaye et al. [34] conducted a study where they identified Staphylococcus aureus in 37 out of 121 milk samples with mastitis, accounting for 30.6% of the total isolates. Among these isolates, 12 (32.4%) were identified as methicillin-resistant Staphylococcus aureus (MRSA) strains. From this finding, they further observed that 5 strains exhibited resistance to two or more beta-lactam antibiotics.
Similarly, Jiménez et al. [9] conducted a study on resistance profiles of Staphylococcus aureus, where they isolated 36 strains. They found that 92.3% of the isolates were sensitive and 7.7% were resistant to 1 µg oxacillin.
In addition, they observed that 95.4% of the isolates were sensitive to cefoxitin, while 4.6% exhibited resistance. These findings differ from the cited publications, as the present study reported a prevalence of 32% of methicillin-resistant Staphylococcus aureus.
Conclusion
In susceptibility tests of Staphylococcus aureus, it is recommended to use amoxicillin and ampicillin at a concentration of 10 µg, as the cutoff points for these drugs are calibrated at this concentration. In the present study, it was found that over 50% of the isolated Staphylococcus aureus strains exhibited resistance to this concentration. Similarly, when these two antibiotics were tested at a concentration of 2 µg, resistance was observed in 50% of the isolates included in the study. The prevalence of methicillin-resistant Staphylococcus aureus (MRSA) was estimated to be 32% based on the antimicrobial activity analysis.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
The authors would like to thank the Ministry of Higher Education (Malaysia) for funding this study through the FRGS/1/2018/STG01/UKM/02/20 grant. They would also like to thank the Faculty of Health Sciences, Universiti Kebangsaan Malaysia (UKM), and the Environmental Health and Industrial Safety Programme for providing the facilities to conduct this study. Special gratitude is given to the management and lab assistants of the Biocompatibility and Toxicology Laboratory of the Centre of Toxicology and Health Risk Studies of Universiti Kebangsaan Malaysia for the assistance provided throughout this research.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Orcid
Riveliño Ramon-Curay
https://orcid.org/0000-0001-6284-4223
Darwin Robalino Salas
https://orcid.org/0009-0009-0080-0271
Nelly Tacle García
https://orcid.org/0009-0004-3886-5584
Jenny Martínez Moreira
https://orcid.org/0000-0001-5445-9800
Favian Bayas-Morejón
https://orcid.org/0000-0003-2920-7155
HOW TO CITE THIS ARTICLE
Favian Bayas-Morejón*, Darwin Robalino Salas, Nelly Tacle García, Riveliño Ramon-Curay, Jenny Martínez Moreira. Antimicrobial Effectiveness of Methicillin, Amoxicillin and Ampicillin against Methicillin-Resistant Staphylococcus Aureus (MRSA) Strains Isolated from Bovine Mastitis. J. Med. Chem. Sci., 2023, 6(11) 2608-2619.


