Document Type : Original Article
Authors
Biology Department, Universitas Negeri Surabaya, Surabaya, Indonesia
Abstract
Background: Bruguirea gymnorrhyza is used to treat some diseases traditionally. Some scientific studies are important for its explanation and drug development. The aims of this study are to identify metabolites in B. gymnorrhyza leaf extract and predict their oral bioavailability and GI absorption associated with treating diseases.
Methods: B. gymnorrhyza leaves were dried. Its flour was macerated in ethanol, then evaporated to produce an extract. The extract was analysed using Liquid Chromatography High Resolution-Mass Spectrophotometry (LCHR-MS) technique to identify its metabolites. The SwissADME tool was applied to predict their oral bioavailability and gastrointestinal (GI) absorption.
Results: The LCHR-MS analysis resulted that B. gymnorrhyza leaf extract contained choline (65.18%) as the most dominant metabolite, followed by diisobutylphthalate (7.23%), isoleucine (5.62%), 2,2,6,6-tetramethyl-1-piperidinol (TEMPO) (4.18%), valine (3.52%), l-phenylalanine (2.07%), d-(+)-proline (1.70%), 2-[(3S)-1-(cyclohexylmethyl)-3-pyrrolidinyl]-1H-benzimidazole-5- carbonitrile (1.44%), adenine (1.39%), 2-[(2-chlorobenzyl)sulfanyl]-4,6-dimethylnicotinonitrile (1.26%), l-norleucine (1.24%), dibenzylamine (1.26%) and wogonin (1.05%). Most of them were predicted not to be oral bioavailable, except diisobutylphthalate, TEMPO, 2-[(3S)-1-(Cyclohexylmethyl)-3-pyrrolidinyl]-1H-benzimidazole-5-carbonitrile, and wogonin. However, most of the metabolites were predicted to be absorbed by GI wall highly, except choline.
Conclusion: The B. gymnorrhyza leaf extract contains 13 main metabolites dominated by choline. They are mostly predicted not to be oral bioavailable and highly absorbed by GI tract. These results can be referred to direct and determine further steps of scientific studies of B. gymnorrhyza leaf extract and its metabolites as disease treating agents.
Graphical Abstract
Keywords
Introduction
Diseases treatments, particularly degenerative ones, require relatively high costs. As a result, people in developing countries use parts of plants as alternative medicines. B. gymnorrhyza is a coastal plant being very important for keeping soil to prevent coastal erosion [1]. It mainly grows in tropical areas [2]. In addition, this plant has economic, physical, and ecological functions. One of the economic roles is its function as medicine [3]. As a medicinal plant, it can treat diabetes mellitus, liver disorder, and tumors. Its stems, bark, leaves, fruits, and roots are alternative parts used [4]. Harvesting medicinal plants, especially getting stems and roots, causes exploitation of natural sources. Leaves substitution can be a good management to maintain the plant existence [5]. Accordingly, this study focused on the plant leaves.
Along with the development of science and technology, the herbal medicines have been being scientifically studied. Some studies have been focusing on their active compounds, secondary [6], as well as primary ones [7]. The occurrence of diseases relates to the change of molecular pathways [8]. The change can be reversed or stopped by binding other molecules, such as plant extract metabolites. Accordingly, the molecules can be potential to be drug candidate. Surabaya, East Java, is a coastal area. One of its plants is B. gymnorrhyza. As one of medicinal plants, some screenings identified that B. gymnorrhyza extract acted as antioxidant [9, 10], anti-inflammatory [11], and antibacterial [12] agents. Several studies showed that the extract contained polyphenols, phenolic, and flavonoid groups, vanillic acid [10], and tannins [13]. Partially, Sadeer and colleague isolated cryptochlorogenic acid from the plant [14]. Some previous works also examined that B. gymnorrhyza functioned as hepatoprotective and ulcerative colitis protective agents [11]. Referring to Madariaga-Mazón and his team, some plants contained metabolites being potential for treating diseases [15]. However, some of them can be harmful, particularly in high doses [16]. In this case, the metabolites’ individual type is important to be identified.
Soil content is closely related to the accumulation of plants’ metabolites [17]. Different places possess different soil compositions [17, 18]. Consequently, the plants’ metabolite content can be different.
To be an effective drug, a potential molecule might reach its target in sufficient concentration. To meet this need, the compound should be bioavailable and easily absorbed by gastrointestinal (GI) wall [19] to reach circulatory system [20]. The type of drug administration determines its route to get the target in the body [19]. Oral administration is the most common manner to be applied because of its convenience. This procedure is also low cost of production and high safety [21]. Prior to laboratory examination, carrying out an in silico method to predict the bioavailability of a molecule is necessary. Based on the molecular properties, the method serves logical predictions of the metabolites’ oral bioavailability and GI absorption. This represents the prediction of the metabolites transports effectiveness to reach the body system targeted [20]. The predictions become crucial in directing further stages to reduce research time and cost. Accordingly, it is important to be applied for the oral bioavailability and GI absorption of each metabolite. The results can be used to consider and determine the further strategy of scientific examination [19]. Based on the illustration, this study aimed to identify the type of metabolites contained in the leaf extract of B. gymnorrhyza and predict their oral bioavailability and GI absorption in relation to their role in treating diseases. Their oral bioavailability and GI absorption predictions based on their molecular properties can direct the further studies, especially to develop alternative strategies to treat diseases.
Materials and Methods
Extraction of plant leaves
The leaves of B. gymnorrhyza were dried to get its simplicia, then crushed into flour. The flour was macerated in 96% ethanol for 24 hs. This step was repeated three times. The sample was then filtered by filter paper. The filtrate resulted was evaporated using a rotary evaporator to produce a semi-solid mass. The last was then stored at -20 ᵒC.
Metabolite identification
The extract was analyzed using a Liquid Chromatography High Resolution Mass Spectrometry (LC-HRMS) system. Before processing, it was diluted in ethanol (polar solvent) at a moderate density with a final volume of 1500 µL. The solution resulted was vortexed in approximately two mins at 2000 rpm. The result was spined down during two mins at 6000 rpm. Its supernatant was taken and filtered using a 0.22 µm syringe filter, and then put into a vial. The analysis was carried out in a Thermo Scientific Dionex Ultimate 3000 RSLCnano device equipped with a nano pump with a microflow meter, vacuum degasser, and thermostatic autosampler. A total of 10 µL of the solution was injected into a column with a temperature of 30 ᵒC the solution was separated on Hypersil GOLD aQ 50 × 1 mm × 1.9 µ particle size. The mobile phases were 0.1% formic acid in water and 0.1% formic acid in acetonitrile. The elution gradient lasted for 30 mins, at a flow rate of 40 L/min. The metabolites were identified using a compound discoverer software with mzCloud Best Match of ≥ 90%. Dominant metabolites contained in concentration of 1% and above then were predicted for their oral bioavailability and GI absorption.
Oral bioavailability and GI absorption predictions
Thirteen dominant metabolites (≥ 1% each) were predicted for their oral bioavailability and GI absorption using SwissADME web tool (http: //www. http://www.swissadme.ch/). The canonical simplified molecular-input line-entry system (SMILES) of each metabolite was got from Pubchem website. If the SMILES was not provided in the website, it was created by drawing the metabolite’s structure in the tool panel provided, and then it was converted into SMILES. The result was put into the SwissADME submission page. By clicking the “Run” button, the tool calculated and showed the levels of oral bioavailability properties ((molecular weight), topological polar surface area (TPSA), aqueous solubility (logS), the mixing character of one 2s-orbital and three 2p-orbitals to create four hybrid orbitals with similar characteristics (sp3), rotatable bond, and the logarithm of the partition coefficient between n-octanol and water (XLOGP3)) of the compound. At the same time, the prediction of their GI absorption also appeared. The molecular characteristics (molecular size, polarity, solubility, saturation, flexibility, and lipophilicity) were visualized in a bioavailability radar. The pink areas represented their optimalities [19]. The same procedure was repeated twice.
Results and Discussion
Metabolite content in the extract
LC-HRMS analysis resulted a list of metabolites content in the leaf extract of B. gymnorrhyza. The main compounds (their concentration were more than 1%) are indicated in Table 1.
There were 13 main metabolites (≥ 1% each) contained in the extract of B. gymnorrhyza. Eleven metabolites belong to the groups of vitamin (choline) (65.18%), phthatalate (diisobutylphthalate) (7.23%), free radicals (2,2,6,6-Tetramethyl-1-piperidinol (TEMPO)) (4.18%), essential amino acids (isoleucine (5.62%), valine (3.52%), l-phenylalanine (2.07%), and d-(+)-proline (1.70%)), base (adenine) (1.39%), amine (dibenzylamine) (1.06%), and flavonoid (wogonin) (1.05%). The others were 2-[(3S)-1-(Cyclohexylmethyl)-3-pyrrolidinyl]-1H-benzimidazole-5- carbonitrile (1.44%) and 2-[(2-chlorobenzyl)sulfanyl]-4,6-dimethylnicotinonitrile (1.26%). Among the compounds, choline was the most dominant. It occupied more than one half part. Determining a plant extract as a drug candidate should consider its metabolites’ content and how the metabolites work. Garcia et al. examined that choline could be an immunomodulator through regulating immune cells [7]. In addition, it played an important role in preventing organ damage and maintaining organ functions, such as by its involvement in metabolism and transports of lipids and cholesterol [22]. In the liver, choline was converted to be betaine, phosphatidylcholine, and acetylcholine [23].
Betain functions as a methyl donor supporting methionine and S-adenosylmethionine. It was also a dietary inhibitor of ROS production. Furthermore, the compound reduced the abundance of cytokine mRNA and enzymes involved in proinflammatory responses in tumorigenic tissues. Phosphatidylcholine was a main component of cell membrane and an effective immunomodulator in vivo. In addition, acetylcholine was the main compound in the cholinergic system involved in the activity of the immune system [7]. Diisobutyl phthatalate is an ester phthatalate. Some studies identified its effects on immune system. It stimulated the system in adjuvant manner [24], influenced by both stereochemical and physicochemical properties [25]. On the other hand, the metabolite possessed some harmful effects, such as causing hypersensitivity [16] and being toxic on macrophage. Using the extract of B. gymnorrhyza, its contribution to some diseases [24] should be considered, particularly for a high dose administration [26]. Adenine is a type of purine base. This metabolite is important for some biochemical structures and mechanisms. Depletion of one of its certain derivatives caused excessive deoxyribonucleic acid (DNA) damage by free radical or the UV attacks. These leaded to inflammatory response [27]. On the other hand, its high dose and excessive duration exposure induced renal impairment [28]. Dibenzilamine (1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-N-(3-fluoro-4-methoxybenzyl)ethan-1-amine) is an antiviral agent. It acted in early step of viral replication by blocking viral RNA and protein synthesis [29]. Wogonin is a type of flavonoid that could treat diseases. The compound worked as anti-neurodegenerative, antiviral, antioxidative, chondroprotective [30], neuroprotective, and anti-inflammatory [31] agents. The two other metabolites 2-[(3S)-1-(Cyclohexylmethyl)-3-pyrrolidinyl]-1H-benzimidazole-5- carbonitrile and 2-[(2-chlorobenzyl)sulfanyl]-4,6-dimethylnicotinonitrile have a limited information about their involvement in treating diseases. Most previous studies on B. gymnorrhyza leaves identified beneficial metabolites, such as flavonoid, phenols, hydroquinones, tannins, saponins, terpenoids, reducing sugars, alkaloids, steroids, terpenoids, and glycosides [4].
Table 1: The main metabolites of B. gymnorrhyza leaf extract

Table 2: Predictions of oral bioavailability properties and GI absorption of metabolites content in B. gymnorrhyza leaf extract
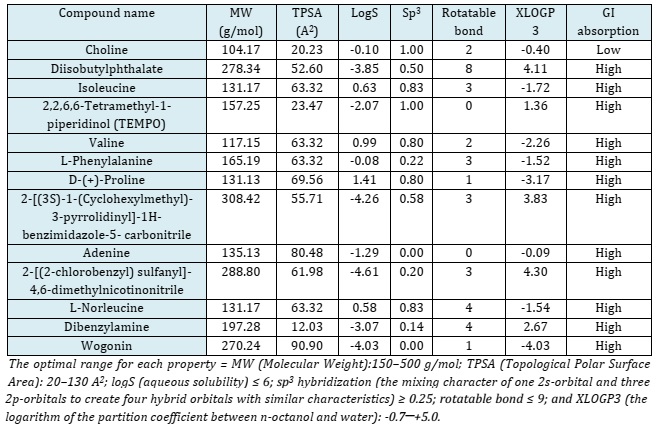
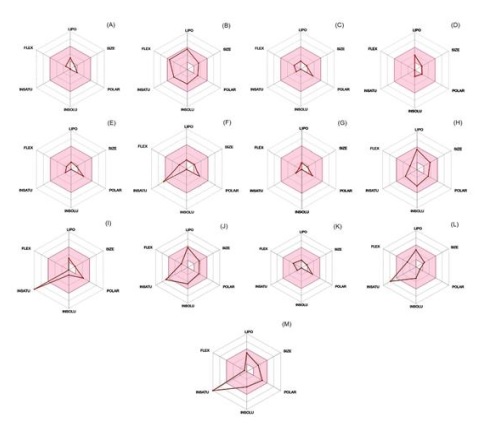
Figure 1: The bioavailability radar of metabolites of B. gymnorrhyza leaf extract: (A) Choline, (B) Diisobutylphthalate, (C) Isoleucine, (D) 2,2,6,6-Tetramethyl-1-piperidinol (TEMPO), (E) Valine, (F) L-Phenylalanine, (G) D-(+)-Proline, (H) 2-[(3S)-1-(Cyclohexylmethyl)-3-pyrrolidinyl]-1H-benzimidazole-5-carbonitrile, (I) Adenine, (J) 2-[(2-chlorobenzyl)sulfanyl]-4,6-dimethylnicotinonitrile, (K) L-Norleucine, (L) Dibenzylamine, and (M) Wogonin. Pink area represents optimal range for each property. SIZE= size of molecule represented by molecular weight; POLAR = polarity; INSOLU = insoluble; INSATU = insaturation; FLEX = flexibility; and LIPO = lipophilicity
However, the recent research found some harmful compounds. This finding can improve the management of the herbal treatment.
Oral Bioavailability and GI absorption predictions
The results of the oral bioavailability and GI absorption predictions can be seen in Table 2. Furthermore, their oral bioavailability is visualized in Figure 1.
A compound is predicted bioavailable orally if it possesses 150 g/mol-500 g/mol molecular weight, 20 Ų─30 Ų TPSA, ≤ 6 log S, ≥ 0.25 sp3, ≤ 9 rotatable bound and -0.7+5.0 XLOGP3 [19]. Running the tool after plotting canonical SMILES in SwissADME panel, choline had 104.17 g/mol molecular weight, 20.23 Ų TPSA, 1.00 sp3, 2 rotatable bound, -0.10 Log S and -0.40 XLOGP3. The molecular weight seemed to be in the outside of the optimum range confirmed by bioavailability radar (Figure 1a). Its GI absorption was also low (Table 2). Hence, the compound was predicted not to be bioavailable and difficult to reach circulatory system.
According to its properties, the molecular size is related to the molecule’s movement across the cell membranes in a passive transport. Rotatable bond count increases with molecular weight [32]. Polarity (represented by TPSA) is used to predict the membrane permeability and drug candidate solubility [33]. Water solubility is important for drug to be absorbed by GI in the aspect of its dissolution in intestinal transit time to reach the surface of absorptive cells. The high water solubility affects the drug to be difficult to be absorbed passively through lipid cell membrane [34]. Hence, the drug should be lipophilic. Saturation is the fraction of carbons in the sp3 hybridization [19]. The increase of saturation indicates the increase the compound’s solubility through the decrease of melting point [35]. In addition to membrane permeation, lipophilicity, molecular weight and hydrogen-bonding potential are related to drug metabolism, especially CYPs’ functions [33]. All bioavailability parameters of diisobutylphthatalate were in optimum ranges (Table 2). It had 278.34 g/mol molecular weight, 52.60 A2 TPSA, -3.85 LogS, 0.50 of sp3, 8 rotatable bonds, and 4.11 XLOGP3. The radar also plotted the parameters in the pink area (Figure 1b). These were supported by the high GI absorption (Table 2). It means that the metabolite was predicted bioavailable orally and easy to reach circulatory system. All essential amino acids possessed two parameters being not optimal. Isoleucine, valine, d-(+)-proline, and l-norleucine had molecular weights of 131.17, 117.15, and 131.13 g/mols, respectively. In addition, their XLOGP3s were -1.72, -2.26, -3.17, and -1.54, consecutively. L-phenylalanine showed 0.22 sp3 and -1.52 XLOGP3. They were outside the pink area of the radar (Figures 1c, e, g, k, and f). However, all of the acids were highly absorbed by GI wall (Table 2). Accordingly, the compounds were predicted not to be orally bioavailable. On the other hand, the GI wall facilitated their movement to reach the circulatory system. Besides diisobutylphthatalate, all parameters of oral bioavailability (molecular size, poparity, insolubility, saturation, flexibility, and lipophilicity) of TEMPO and 2-[(3S)-1-(Cyclohexylmethyl)-3-pyrrolidinyl]-1H-benzimidazole-5- carbonitrile were optimum (Table 2). Figure 1d and h exhibit them in the pink area. Their GI absorptions were also predicted to be high (Table 2).
The three last metabolites bioavailability parameters were outside the optimum ranges (Table 2). Adenine had molecular weight of 135.13 g/mol and sp3 of 0.00. The nonoptimal parameter of dibenzylamine was for sp3 (0.14). Meanwhile, wogonin possessed sp3 of 0.00 and XLOGP3 of -4.03. These data matched with their radars. They exhibit some parameters outside the pink area, i.e. adenine (size and instauration), dibenzylamine (insaturation), and wogonin (insaturation). Contrary, their GI absorptions were high (Table 1). It means that the metabolites were not bioavailable orally, however, their properties supported to pass the GI wall. Besides the predictions, some previous examination results can strengthen the further logical step decisions. Choline was the most dominant content in the extract of B. gymnorrhyza leaf. The compound possesses functions relating to health treatments [22]. However, its oral bioavailability and GI absorption were predicted to be low (Table 2). Tsubaki and his colleague examined that choline was absorbed very rapidly from the lumen, attaining 97% for 30 mins [36]. On the other hand, the rate reduced at the longer time and higher concentrations. These phenomena indicated an involvement of its saturation process during choline transportation from lumen across the GI wall to reach the circulatory system. Accordingly, although the compound supply was high (Table 1), the GI tract condition limited its access to the circulatory system. This supports its prediction, being not orally bioavailable (Table 2 and Figure 1a). Sharifi-Rad et al. suggested combining with other metabolite/s or using nanotechnology tools to enhance the bioavailability [30]. Diisobutyl phthatalate [16] and TEMPO [37] were harmful and contribute to some diseases. Furthermore, they are predicted to be bioavailable orally and highly absorbed by GI wall (Table 2). Their low content (Table 1) and low dose may be saver for the whole extract of B. gymnorrhyza leaf application. This study predicted that the essential amino acids were not orally bioavailable (see Table 2 and Figure 1c, e, f, g, and k). This was due to their molecular weighs, lipophilicity, or saturation being not in the optimum area. It seems that the molecules have problems to reach circulatory system. However, Levesque and team found that the brush border of the small intestine contained human peptide transporter 1 (hPEPT1) mediated the acids passing through the cell membrane [38]. Dibenzilamine (1-(2, 3-dihydrobenzo[b][1,4]dioxin-6-yl)-N-(3-fluoro-4-methoxybenzyl) ethan-1-amine) [28,39,40] and wogonin [30-31] were capable to treat diseases. Although they were predicted to be bioavailable orally and highly absorbed by GI tract, their concentration in the extract of B. gymnorrhyza was low (Table 1). Eid and his team suggested to consider the existence of other compound/s having synergistic effects increasing the mechanisms [6]. Two other compounds,
2-[(3S)-1-(Cyclohexylmethyl)-3-pyrrolidinyl]-1H-benzimidazole-5- carbonitrile and 2-[(2-chlorobenzyl)sulfanyl]-4,6-dimethylnicotinonitrile, have limited information about their function related to disease treatment. They were predicted to be bioavailable orally (Table 2) and highly absorbed by GI tract. However, their content was relatively low (< 2%) (Table 1). It seems to have low effect on cells. Even though, for initial information, predicting and examining whether they are toxic on normal cells or not are necessary [41]. Referring the results of this work and some previous studies, some controversies of metabolites contained in B. gymnorrhyza for treating diseases appear. The dominant metabolite was initially capable to treat diseases, but its oral bioavailability and GI absorption were predicted to be not support the function. Second, some metabolites were harmful. On the other hand, they were predicted to be oral bioavailable and highly absorbed by GI tract. Third, some compounds were capable to treat diseases; however, their content was low. Developing drug needs some strategies and methods to ensure that the drug would be effective and save for the patients, besides a low-cost consuming. Using plant extract can be as whole extract or partial metabolites. The initial manner mainly refers to the dominant compound [42]. As a whole sample, the mechanism in treating diseases using B. gymnorrhyza leaf extract can be focused on choline functions as immunomodulator, neurodegenerative, DNA synthesis and repair, and maintenance of cell structure agents. Other metabolites [6], such as amino acids, adenine, dibenzylamine and wogonin, may reinforce the functions each other. Relating to their oral bioavailability properties, GI absorption, and harmfulness, some strategies can be applied to overcome the controversies. Initially, using the whole extract refers to the dominant metabolites [42]. The second is using an individual metabolite isolated from the extract. The third is combining metabolites to improve their bioavailability [43]. The fourth is using nanotechnology tool to enhance its metabolites’ oral bioavailability. Furthermore, the existence of transporter to in the GI wall, such as hPEPT1 [38], is also important to plan a strategy. However, this study only examined the leaf extract of B. gymnorrhyza from Surabaya, Indonesia. The content and potential of B. gymnorrhyza from other place might be different.
Conclusion
This study identified that the leaf extract of Bruguirea gymnorrhyza contained various metabolites that most of them were predicted not to be oral bioavailable, except diisobutylphthalate, TEMPO, 2-[(3S)-1-(Cyclohexylmethyl)-3-pyrrolidinyl]-1H-benzimidazole-5-carbonitrile and wogonin. However, most of the metabolites were predicted to be absorbed by GI wall highly, except choline. Further research can consider the use of the extract either as a whole extract or isolated metabolites. In addition, their quantitative contents, level of toxicity or harmfulness, and strategies of administration of B. gymnorrhyza can be examined or applied.
Acknowledgements
The authors would like to thank Universitas Negeri Surabaya for financial support. They also extend their gratitude to The Faculty of Mathematics and Natural Sciences of Universitas Negeri Surabaya that have facilitated in getting scientific assistance in writing the manuscript. Likewise, they would like to thank to the reviewers who give suggestions to improve the quality of this manuscript.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
ORCID
Nur Kuswanti
https://orcid.org/0000-0001-9445-6954
Nur Qomariyah
https://orcid.org/0000-0003-0003-1870
Erlix Rakhmad Purnama
https://orcid.org/0000-0002-4066-3357
Firas Khaleyla
https://orcid.org/0000-0002-2744-9364
HOW TO CITE THIS ARTICLE
Nur Kuswanti*, Nur Qomariyah, Erlix Rakhmad Purnama, Firas Khaleyla, Bruguirea gymnorrhyza Leaf Extract Metabolites: Oral Bioavailability and GI Absorption Predictions. J. Med. Chem. Sci., 2024, 7(3) 518-529.
Toxicological Sciences, 2018, 163:214 [Crossref], [Google Scholar], [Publisher]