Document Type : Original Article
Authors
- Oggy Satriya Putra 1
- Luki Indriaswati 1
- Yulia Primitasari 1
- Nandang Sudrajat 1
- Bimanda Rizki Nurhidayat 1
- Mahmudah Mahmudah 2
- Thomas Valentinus Widiyatno 3
1 Department of Ophthalmology, Faculty of Medicine, Airlangga University, Dr. Soetomo General Academic Hospital, Surabaya, Indonesia
2 Department of Public Health and Preventive Medicine Medical Faculty of Airlangga University, Surabaya, Indonesia
3 Department of Veterinary Pathology, Faculty of Veterinary Medicine Airlangga University, Surabaya, Indonesia
Abstract
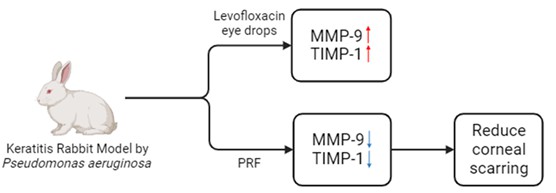
Background: Pseudomonas aeruginosa is the most common cause of bacterial keratitis, with potential complications such as corneal perforation and endophthalmitis. Platelet-rich fibrin (PRF) membrane is utilized as a treatment for corneal ulcers. Maintaining a balance between collagen formation and extracellular matrix remodeling is crucial in fibrosis, a process regulated by matrix metalloproteinases (MMPs) and Tissue Inhibitors of Matrix Metalloproteinases (TIMPs). This study aims to analyze the effect of the PRF membrane on the expression of MMP-9 and TIMP-1 in keratitis.
Methods: This study employed an experimental design with a randomized posttest-only control group. New Zealand white rabbits (Oryctolagus cuniculus) were used as animal models with Pseudomonas aeruginosa keratitis. Levofloxacin eye drops were administered to both the control and treatment groups. However, in the treatment group, a PRF membrane was sutured. The expression of MMP-9 and TIMP-1 was compared between the two groups.
Results: The animal models consisted of 12 New Zealand white rabbits, divided into a control group and a treatment group, each comprising 6 rabbits. The mean ± SD of MMP-9 in the treatment group was 2.58 ± 0.72, whereas in the control group, it was 4.62 ± 1.56. TIMP-1 data in the treatment group had a median of 4.10 (with a minimum of 2.00 and a maximum of 4.80), while in the control group, it was 4.80 (with a minimum of 4.20 and a maximum of 8.96). Analysis using the independent t-test and Mann-Whitney test revealed substantial disparities in the expression levels of MMP-9 and TIMP-1 between the control and treatment groups, with p-values of 0.016 and 0.036, respectively. The treatment group exhibited a lower expression of MMP-9 compared to the control group, whereas the treatment group demonstrated a higher expression of TIMP-1 in comparison to the control group.
Conclusion: PRF membranes can modulate MMP-9 expression and the balance between MMP-9 and TIMP-1 in degrading and depositing the extracellular matrix (ECM). They can be considered as an option for cases of Pseudomonas aeruginosa keratitis to reduce corneal scarring.
Graphical Abstract
Keywords
Introduction
Pseudomonas aeruginosa is the most common cause of bacterial keratitis, characterized by its invasive and rapid growth nature. This bacterium has the potential to induce severe ocular infections, including endophthalmitis and corneal perforations, leading to compromised vision. In addition, Pseudomonas aeruginosa produces exotoxins S, T, and U, contributing to tissue necrosis [1]. The administration of platelet-rich fibrin (PRF) membrane has emerged as a safe and effective treatment for corneal ulcers with a success rate of up to 89.4% [2, 3]. Despite its efficacy, there is the lack of studies examining the impact of PRF membrane usage on Pseudomonas aeruginosa keratitis and its potential to mitigate chronic complications such as corneal scarring.
Corneal scarring represents a vision-threatening corneal opacities caused by abnormal healing of a corneal defect deep in the stroma, accompanied by an imperfect arrangement of the extracellular matrix (ECM) and collagen fibers [4,5]. Globally, approximately 40 to 45 million individuals experience blindness, with 4% of cases attributed to corneal opacities.
The World Health Organization (WHO) reports 1.5 to 2 million new cases of blindness annually due to corneal opacities and infection-associated neovascularization [6]. In the 2014 Rapid Assessment of Avoidable Blindness (RAAB) survey in Indonesia, non-trachomatous corneal opacities accounted for 4.3% of total blindness cases [7]. Notably, Pseudomonas aeruginosa is a prevalent pathogen causing bacterial keratitis, accounting for 21% of cases in the United States and 24.7% in Indonesia [8, 9]. Data from Cipto Mangunkusumo Public Hospital in Jakarta indicates that Pseudomonas aeruginosa is responsible for 25% of keratitis cases [8]. Meanwhile, at Dr. Soetomo Public Hospital in Surabaya, it is identified as the second most common cause of keratitis [10, 11].
During the process of wound healing and tissue repair, cells continuously synthesize the ECM. The healing phase of the corneal stroma involves the synthesis, breakdown, and cross-linking of collagen, resulting in the formation of strong stromal tissue. Regeneration and maintenance of the corneal stroma represent major functions of keratocytes.
Keratocyte activation leads to either apoptosis or proliferation, causing keratocytes to migrate to the wound area and transform into fibroblasts. Activated fibroblasts further transform into myofibroblasts, critical cells in the wound-healing process [12].
Myofibroblasts, derived from keratocytes, are crucial in triggering transforming growth factor-beta (TGF-β), believed to stimulate scar tissue formation across the body, including the eyes. However, myofibroblasts act as a double-edged sword; while useful in wound healing, excessive presence can lead to fibrosis and subsequent scarring.
The delicate balance between collagen assembly and ECM remodeling is vital in the fibrosis process, regulated by matrix metalloproteinases (MMPs) and their inhibitors, Tissue Inhibitors of Matrix Metalloproteinases (TIMPs), both produced by myofibroblasts. In the corneal fibrosis process, normal tissue repair can progress to progressively irreversible fibrosis due to the excessive transformation of fibroblasts into myofibroblasts, resulting in increased collagen deposition [13-15]. Platelet-rich fibrin (PRF) is an autologous growth factor derived from platelet-rich blood, containing cytokines and growth factors that accelerate the wound-healing process. The manufacturing process of PRF does not require bovine thrombin and anticoagulants, making it relatively inexpensive and advantageous in the regenerative field. A study by Demir et al. demonstrated that PRF membranes were quite safe and effective in managing corneal ulcers in cats, with a success rate of 89.4% [2]. Currently, no studies examine the effect of using PRF membranes on Pseudomonas aeruginosa keratitis. This study aims to analyze the impact of PRF membranes on infectious keratitis induced by Pseudomonas aeruginosa by assessing the expression of MMP-9 and TIMP-1, essential components of the wound healing mechanism.
Materials and Methods
This study employed an experimental design with a randomized posttest-only control group. New Zealand white rabbits (Oryctolagus cuniculus) were used as experimental animal models. The inclusion criteria for this study encompassed male New Zealand white rabbits (Oryctolagus cuniculus) aged between 4 and 10 months. These rabbits were required to have a weight between 3,000 and 3,500 grams, exhibit overall good health and activity levels, and have healthy surfaces of the eyeballs. In addition, their platelet count needed to be within the range of 390-821 X 109/L, while the fibrinogen value should be within the range of 1.66 ± 0.39 g/L [16].
The exclusion criteria encompassed rabbits exhibiting ocular disorders and other diseases with potential inter-rabbit transmission. Diagnoses were conducted by trained veterinarians. The drop-out criteria encompassed rabbits that fell sick, died, or experienced complications (e.g., conjunctival infection and bleeding) during and after the induction procedure. All rabbits underwent examination by trained veterinarians on day 1. The same type and amount of food and water were provided to all rabbits three times a day at consistent times. Bacterial keratitis was experimentally generated by mechanically abrading the corneal epithelium, followed by the application of a suspension containing Pseudomonas aeruginosa on the first day of the study. The rabbits were administered intramuscular injections of ketamine hydrochloride at 30 mg/kg and xylazine hydrochloride at 5 mg/kg for anesthesia. Corneal defects of up to 10 mm were created using the deep scratch technique employing a sterile 21G needle. A suspension (0.3 ml) containing 5 X 108 CFU/ml of Pseudomonas aeruginosa was instilled onto the scratched cornea. The animal models were divided into two groups: the control group and the treatment group. Levofloxacin 0.5% eye drops were administered to all rabbits in both groups starting from day 3, 48 hours after the induction procedure, and continued until day 10. The dosage administered was 1 drop four times a day [17]. Platelet-rich fibrin (PRF) membranes were prepared from blood samples collected from each rabbit in the treatment group. Blood samples (5 ml) were collected from each rabbit's auricular veins and stored in containers without any anticoagulants. The samples were then centrifuged at 2,700 rpm for 12 minutes. After centrifugation, erythrocytes would be at the bottom, concentrated fibrin clots would be in the middle, and acellular plasma or platelet-poor plasma (PPP) would be at the top of the tube. After removing the PPP, the fibrin clots were mechanically separated from the erythrocytes using forceps. Subsequently, the clots were compressed using a PRF box, a specifically designed membrane-shaped apparatus employed to drain excess fluid from the platelet-rich fibrin (PRF).
PRF membranes were then cut into sizes appropriate for the cornea and sutured using an overlay technique onto the induced corneas of rabbits in the treatment group on day 4, 24 hours after the first levofloxacin eye drops were administered.
Partial-thickness sutures were performed using 10-0 nylon thread at four locations: superior, inferior, nasal, and temporal, approximately 0.5-1 mm from the limbus. Levofloxacin eye drops were continued for both groups until day 10. The study termination was conducted on day 10. All rabbits were enucleated to examine the expression of MMP-9 and TIMP-1 using immunohistochemistry tests with MMP-9 and TIMP-1 antibody kits. The collected data were recorded and tabulated. Data processing in this study utilized SPSS software. The analysis was performed using the independent t-test and Mann-Whitney test, employing a significance level of 5%.
Results and Discussion
This study utilized a sample size of 12 New Zealand white rabbits, which were divided into two distinct groups: the control group and the treatment group, each consisting of 6 New Zealand white rabbits. All samples met the inclusion criteria, and no samples were excluded or dropped out. None of the samples exhibited illness, mortality, or complications. MMP-9 and TIMP-1 data were recorded, and normality tests were conducted using the Shapiro-Wilk test. The data pertaining to MMP-9 demonstrated a normal distribution, and the results were reported as mean and standard deviation. However, the distribution of TIMP-1 data deviated from normality, leading to the presentation of summary statistics such as median, the minimum, and the maximum values. The mean ± SD of MMP-9 in the treatment group was 2.58 ± 0.72, while in the control group, it was 4.62 ± 1.56 (Table 1). The application of the independent t-test revealed a statistically significant difference in MMP-9 expression levels between the control group and the treatment group.
The TIMP-1 data in the treatment group exhibited a median of 4.10 (with a range of a minimum of 2.00 to a maximum of 4.80), whereas, in the control group, the median was 4.80 (with a range of a minimum of 4.20 to a maximum of 8.96) (Table 2). Utilizing the Mann-Whitney test, substantial statistically significant disparities were observed in the TIMP-1 expression between the control group and the treatment group.
Infectious keratitis is a prevalent form of keratitis and a significant cause of global blindness. The clinical manifestations of infectious keratitis vary based on the causative pathogens. The clinical signs of keratitis resulting from Pseudomonas aeruginosa are more severe compared to those associated with other forms of bacterial keratitis. [18]. The primary approach to managing infectious keratitis involves controlling infection, addressing inflammation, and facilitating the healing process of the cornea. If prompt and appropriate therapy is not received, complications may occur, which may result in blindness, including scarring of the cornea [19, 20].
Table 1: MMP-9 expression data
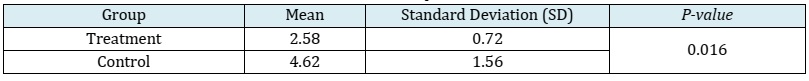
Table 2: TIMP-1 expression data

In the presence of corneal tissue damage during keratitis, inflammatory cells produce cytokines that activate keratocytes. This activation results in an upregulation of MMP expression during the wound-healing phase. MMPs are enzymatic proteins crucial for degrading the extracellular matrix. Fibroblasts synthesize these enzymes in the early stages of wound healing, contributing to tissue remodeling. The MMP family comprises various subgroups, including collagenase (MMP-1, MMP-8, and MMP-13), gelatinase (MMP-2 and MMP-9), stromelysin (MMP-3 and MMP-10), matrilysin (MMP-7 and MMP-26), and membrane-type MMPs bound to the epithelial cell membrane. MMPs play pivotal roles in normal development, reproduction, tissue remodeling, and wound-healing processes. Bacterial keratitis due to Pseudomonas aeruginosa significantly upregulates MMP expression, particularly MMP-1, MMP-3, MMP-8, and MMP-9. MMP-9, in particular, plays a critical role in inflammation and regeneration by recruiting leukocytes and mobilizing progenitors or stem cells. Increased MMP-9 expression is detected in bacterial keratitis, often within the first day after the inoculation of infected tissue. MMP-9 also plays a role in keratocyte migration. In chronic wound conditions, there is an escalation in keratocyte migration and MMP-9 activity. Uncontrolled MMP expression can lead to excessive ECM degradation, fibrotic tissue formation, and corneal neovascularization, ultimately reducing corneal transparency and visual acuity. Conversely, decreased MMP-9 expression in bacterial keratitis plays a protective role for the cornea, preventing excessive ECM degradation [21-30]. MMP-9 expression was examined using the immunohistochemistry with MMP-9 antibodies. The data on MMP-9 expression scores were collected using the Remmele method, which involves calculating the Remmele scale index (Immunoreactive Score/IRS). This index is determined by multiplying the percentage score of cells or areas exhibiting positive immunoreactivity with the color intensity score on immunoreactive cells. In the treatment group, MMP-9 expression significantly increased when compared to the control group. This is consistent with an in vitro study by Eren et al., who examined MMP-9 and TIMP-1 expression in gingival crevicular fluid cells treated with PRF membranes. The study reported that PRF membranes significantly inhibited MMP-9 expression by regulating inflammation and promoting the wound-healing process. This increase in MMP-9 may be attributed to production by TGF-β and other pro-inflammatory cytokines. In an in vitro animal study by McClellan et al., which examined MMP-9 expression in Pseudomonas aeruginosa bacterial keratitis, MMP-9 expression was increased during inflammation with TNF-α, NF-κB, IL-1, TGF-β, and PAF as pro-inflammatory mediators [31-33]. PRF membranes serve as a scaffold for cell proliferation, differentiation, and migration, crucial for cell regeneration. It also helps provide various slow-growth factors such as TGF-β [34]. TGF-β1, an isoform of TGF-β, plays a crucial role in increasing fibroblast chemotaxis and enhancing ECM production by regulating MMP expression and activity in remodeling and wound healing. However, the role of TGF-β1 on MMPs is not yet fully understood. In a study reported by Chen et al., TGF-β1 and TGF-β3 were found to reduce MMP production and increase TIMP-1 production in human lung fibroblasts and myometrial smooth muscle cells. Another study by Palosaari et al. showed that TGF-β1 stimulated the production of MMP-9 in tooth cells (odontoblasts). In a separate study, Kim et al. reported that increased TGF-β1 activity in the corneal epithelium stimulated MMP-9 production, while in the corneal stroma layer, it actually inhibited MMP-9 production [35-39].
In a previous study by Mulholland et al., a significant increase in MMP-9 expression was observed on day 5, followed by a decrease on day 14. Another study by Li et al. also reported a significant increase in MMP-9 expression on days 1, 2, 3, and 6, with a subsequent decrease starting on day 10 and a significant decrease on day 14. However, in this study, MMP-9 expression was calculated on the 7th day after using PRF membranes, and the results demonstrated a significant increase in MMP-9 expression in the PRF membrane group compared to the control group. This increase may be attributed to the manipulation of the cornea on the 3rd day after the keratitis induction, where the researchers sewed the PRF membrane in the treatment group, while in the control group, the PRF membranes were not sutured. Another contributing factor is that MMP-9 expression was examined only on day 1 and on day 7 after using PRF membranes or on day 10 after the induction of keratitis [40, 41]. Tissue inhibitor of metalloproteinase-1 (TIMP-1) serves as an endogenous inhibitor of matrix metalloproteinase-9 (MMP-9), acting as an antagonist to its enzymatic activity. The equilibrium between TIMPs and MMPs is a critical factor in the mechanism of extracellular matrix (ECM) breakdown and deposition. In the initial step, TIMPs form a binding interaction with MMPs, followed by further binding to the catalytic domain of MMPs, facilitating the removal of zinc cations from the active site. This process ultimately disrupts the enzymatic activity of MMPs. The chemicals in question have the ability to induce proteolytic alterations, potentially leading to the development of pathogenic states. The outcome of wound healing is contingent upon the TIMPs concentration, a regulatory factor that modulates the MMPs activity. TIMP-1 secretion is observed in a wide range of cells throughout the body, exerting inhibitory effects on all MMPs except for MMP-14, MMP-16, MMP-18, MMP-19, MT1-MMP, MT2-MMP, MT3-MMP, and MT5-MMP [25, 42, 43].
Under normal conditions or homeostasis, MMPs and TIMPs are in balance. However, infection or inflammation can trigger metabolic and immunological reactions that disrupt this balance, thereby affecting the tissue repair process. In cases of infections such as bacterial keratitis, TIMP-1 acts through two mechanisms. The first is an MMP-dependent mechanism, where TIMP-1 activity is dependent on MMPs. TIMPs effectively inhibit the MMPs activity by maintaining a stoichiometric ratio of 1:1, leading to a reduction in ECM degradation. According to reports, this behavior is considered reversible. Simultaneously, TIMPs also regulate other cellular processes, including cell development, proliferation, apoptosis, migration and angiogenesis, through their interaction with receptors and subsequent activation of certain signaling pathways [44-47]. TIMP-1 expression was examined using immunohistochemistry with TIMP-1 antibodies, similar to how MMP-9 was examined. In this study, TIMP-1 expression was measured, and a significant difference was observed between the two groups. TIMP-1 expression increased in the group given PRF membranes compared to the control group. This finding aligns with a study by Shao et al., which reported an increase in the expression of TIMP-1 and TIMP-2 after PRF administration in skin epithelial defects. PRF given to the skin defect plays a role in assisting cell migration, proliferation, and secretion, as well as producing growth factors that are slowly released. As these growth factors increased, ECM deposition also increased by filling the defect and migrating cells to the defect area. The deposition from the ECM was consistent with an increase in TIMP-1 [48]. Nonetheless, the effect of PRF membranes on TIMP-1 expression has not been extensively studied. According to a study by Eren et al., which examined MMP-9 and TIMP-1 expression in gingival crevicular fluid cells treated with PRF membranes, it was reported that PRF membranes significantly increased the expression of TIMP-1 accompanied by a decrease in MMP-9 expression. This finding aligns with the study by Kornsuthisopon et al., which investigated PRF stimulation on canine periodontal regeneration. Research explains that the TIMP-1expression was significantly increased in the group given PRF [31, 49]. Currently, the main therapy for bacterial keratitis involves topical antibiotics, which are useful for controlling infection and inflammation, as well as helping the corneal healing process. O'Callaghan et al. showed that topical antibiotic therapy could suppress infectious and inflammatory processes, but predicting corneal healing and fibrosis remains challenging [3]. This has an effect on the balance between ECM degradation and deposition, which is modulated by MMPs and TIMPs. The study's findings revealed significant variations in the levels of MMP-9 and TIMP-1 expression in the cornea of the Pseudomonas aeruginosa keratitis animal model, particularly between the control group and the group treated with PRF membranes. In the treatment group, which received both antibiotics and the PRF membrane, the expression levels of MMP-9 and TIMP-1 were observed to be higher compared to the control group, which solely received antibiotics. The application of PRF membranes has the ability to modify the expression of MMP-9 and the balance between MMP-9 and TIMP-1 in the degradation and deposition of the extracellular matrix (ECM). This modulation can potentially decrease the likelihood of corneal fibrosis and may serve as an alternative therapeutic approach for individuals with Pseudomonas aeruginosa keratitis.
Conclusion
The utilization of PRF membranes demonstrates the potential to regulate MMP-9 expression and maintain a delicate equilibrium between MMP-9 and TIMP-1 in the extracellular matrix degradation and deposition processes. Consequently, this approach holds the potential to mitigate the incidence of corneal fibrosis and stands as a viable therapeutic alternative for cases of Pseudomonas aeruginosa keratitis.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
ORCID
Oggy Satriya Putra
https://orcid.org/0009-0006-9924-9528
Luki Indriaswati
https://orcid.org/0009-0000-4904-5013
Yulia Primitasari
https://orcid.org/0000-0003-3531-9858
Nandang Sudrajat
https://orcid.org/0000-0003-1195-1947
Bimanda Rizki Nurhidayat
https://orcid.org/0000-0002-5790-2748
Mahmudah
https://orcid.org/0000-0002-7434-0847
Thomas Valentinus Widiyatno
https://orcid.org/0000-0002-0745-3796
HOW TO CITE THIS ARTICLE
Oggy Satriya Putra, Luki Indriaswati*, Yulia Primitasari, Nandang Sudrajat, Bimanda Rizki Nurhidayat, Mahmudah, Thomas Valentinus Widiyatno, Effect of Platelet-Rich Fibrin (PRF) Membrane on the Expression of Matrix Metalloproteinase 9 (MMP-9) and Tissue Inhibitor of Metalloproteinase 1 (TIMP-1) in Corneal Wound Healing: Pseudomonas aeruginosa Keratitis. J. Med. Chem. Sci., 2024, 7(3) 482-491.