Document Type : Original Article
Authors
1 Department of Anesthesiology and Critical Care, Arak University of Medical Sciences, Arak, Iran
2 Department of Cardiac Surgery, Arak University of Medical Sciences, Arak, Iran
3 Student Research Committee, Arak University of Medical Sciences, Arak, Iran
4 Department of Epidemiology, Arak University of Medical Sciences, Arak, Iran
Abstract
Background: Bleeding is a significant complication of cardiac surgeries, leading to increased blood product utilization, higher rates of reoperation, and mortality. Tranexamic acid is one of the main medications used to control postoperative bleeding in these patients. Therefore, the aim of this study was to compare the effects of intravenous, topical, and oral tranexamic acid in reducing postoperative bleeding after coronary artery bypass grafting (CABG) surgery.
Materials and methods: This clinical trial was conducted on 96 patients scheduled for CABG surgery. The patients were divided into three groups of 32, receiving oral, intravenous, and topical tranexamic acid. After the surgery and administration of the respective medication, the amount of bleeding within 72 hours postoperatively, as well as the amount of packed red blood cells, platelets, and fresh frozen plasma received within 24 hours postoperatively were assessed.
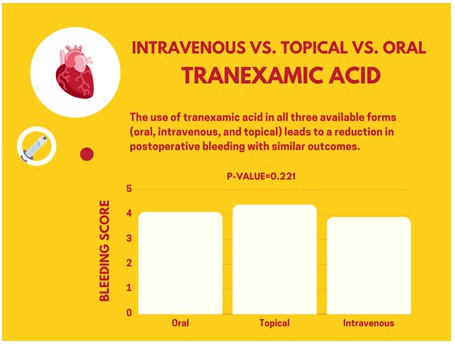
Results: The mean age of the participating patients was 64±5.4 years, and the three groups did not significantly differ in terms of age, gender distribution, number of grafts (P=0.421), and duration of surgery (P=0.624). There was no significant difference in the bleeding score among the three groups, with scores of 3.9±0.88 in the intravenous group, 4.1±1.1 in the oral group, and 4.4±0.98 in the topical group (P=0.221). The average amount of packed red blood cells, platelets, and fresh frozen plasma received during and after surgery was similar in all three groups, with values of 2 (P=0.422), 3.5 (P=0.402), and 2.4 (P=0.412), respectively.
Conclusion: In general, the results of this study indicate that the use of tranexamic acid in all three available forms (oral, intravenous, and topical) leads to a reduction in postoperative bleeding with similar outcomes. Therefore, concerning the fewer side effects of the topical and oral forms and their sufficient effectiveness, physicians can utilize these methods for prescribing tranexamic acid.
Graphical Abstract
Keywords
Main Subjects
Introduction
Acute coronary syndrome manifests in various forms, including unstable angina and myocardial infarction with or without ST-segment elevation. These manifestations stem from coronary artery disease and increase the risk of sudden death and heart attacks [1]. Revascularization and coronary artery bypass graft (CABG) surgery are standard selective treatments for coronary disease.s. They alleviate symptoms and extend life in patients with simultaneous involvement of multiple coronary arteries [2, 3]. However, open-heart surgeries are associated with life-threatening bleeding, leading to higher mortality rates, morbidity, a greater likelihood of returning to the operating room, and an increased need for blood products [4-7]. Heart surgery stands out as one of the procedures with the highest levels of blood product usage and bleeding during and post-surgery. Approximately, 30 to 70 percent of CABG candidates require blood transfusions, prompting attention to antifibrinolytic drugs due to the numerous risks associated with blood product transfusion, including the transmission of HIV and Hepatitis B and C [8, 9]. Approximately, 10% of individuals who are candidates for CABG surgery experience hemorrhagic complications due to the use of antiplatelet drugs [10]. Cardiopulmonary bypass (CPB) is a crucial component of heart surgeries, and before its utilization, patients require antithrombotic drugs, putting them at high risk for bleeding. CPB can disrupt platelet function, reduce clotting factors, and intensify fibrinolysis. About 25 to 40 percent of significant bleeding post-CABG surgery occurs due to fibrinolysis, and even in off-pump surgery, factors such as sternotomy and pericardiotomy can activate the fibrinolytic pathway [11, 12]. Tranexamic acid is one of the antifibrinolytic drugs commonly used in medical practice. This synthetic analog of the amino acid lysine works by irreversibly blocking the conversion of plasminogen to plasmin, thereby preventing the normal function of plasminogen. Moreover, it reduces platelet function and has gained widespread acceptance in medical settings. Moreover, it reduces platelet function and has gained widespread acceptance in medical settings.
The incidence of bleeding among CABG candidate patients due to platelet function inhibition is on the rise, and studies have demonstrated that the use of tranexamic acid in heart surgeries effectively reduces post-operative bleeding. Its efficacy in managing bleeding has led to its incorporation into various medical practices and specialties [13]. Studies have indicated that in cases of glomerular dysfunction, tranexamic acid may accumulate in the body, potentially leading to an increased risk of seizures. It is important to note that patient weight plays a significant role in the drug's pharmacokinetics, and the oral form of the drug has a bioavailability of 46% [14]. While the usage of tranexamic acid has shown some side effects, such as seizures and vascular incidents, it is essential to note that no study has reported a clear impact on increased mortality, morbidity, or heart attacks following the use of tranexamic acid in heart surgeries [15]. With the reduced prescription of aprotinin, tranexamic acid has emerged as the primary treatment to prevent severe bleeding in heart surgeries. Therefore, the aim of the present study is to compare the effects of intravenous, topical, and oral tranexamic acid in reducing bleeding after coronary artery bypass graft (CABG) surgery. This research seeks to shed light on the most effective and safe method of using tranexamic acid to control bleeding in CABG procedures and provide valuable insights for medical practitioners and patients alike.
Materials and Methods
This study was a randomized clinical trial, carried out on patients who were candidates for coronary artery bypass grafting (CABG) surgery and referred to the Emam Hospital in Arak. The patients who met the inclusion and exclusion criteria were enrolled in the study. Inclusion criteria were elective patients who were candidates for CABG surgery, patients classified as ASA 2 and 3, willingness to participate in the study, no history of allergy to tranexamic acid, and no serious underlying diseases such as ESRD, CKD, dialysis patients, chronic pulmonary diseases like COPD, and patients with thyroid disorders. Patients who died for reasons other than bleeding and unwilling to participate were excluded from the study.
Sample size calculation
Based on the formula below, 96 patients who were candidates for CABG were randomly divided into three equal groups using a block randomization method. These groups received local, injectable, and oral tranexamic acid.

The patients included into the study received premedication including 5 mg oxazepam the night before and 3-5 mg of morphine IV and 15-25 mg of promethazine in the morning of the operation. Before entering the operating room, patients in the oral group (A) were given 1 g of tranexamic acid orally (tranexamic acid capsules), 2 capsules of 500 mg with a glass of water. To ensure blindness, the other two groups were given two placebo capsules containing flour, which looked identical to the tranexamic acid capsules in color and appearance, and then 3-5 cc/kg of crystalloid fluid (Ringer's solution) was administered to the patients to compensate for the Compensatory Volume Expansion (CVE). Complete monitoring was set up for patients lying supine on the operating table, including Non-Invasive Blood Pressure (NIBP), Oxygen Saturation (SPO2), Respiratory Rate (RR), Pulse Rate (PR), and Electrocardiogram (ECG). After setting up the monitors, 1-2 cc of fentanyl was given to each patient to establish the Arterial Line for monitoring Invasive Blood Pressure (IBP). The arterial line was inserted into the non-dominant hand's radial artery using a 20-22-gauge needle and connected to the monitor for IBP recording. After setting up the Arterial Line and complete monitoring, the patient was prepared for anesthesia, and induction was performed with 5-10 micrograms/kg of Sufentanil, 0.1 mg/kg of midazolam, and 10-12 mg of pavulon, and then the patients were intubated and, after securing the tracheal tube, were placed under the anesthesia device. Next, patients were prepared to receive a Central Venous (CV) line from the internal jugular vein. After the placement of the three-lumen CV line from the right internal jugular vein, maintenance anesthesia drugs, including muscle relaxants, anesthetics, benzodiazepines, and propofol, were given to patients as Total IV Anesthesia (TIVA). Depending on the patient's hemodynamic stability, they were prepared to be weaned off the heart-lung pump after the completion of the coronary grafts. Before weaning off the heart-lung pump for patients in the local tranexamic acid group (B), 3 g of tranexamic acid was dissolved in 100 cc of normal saline and topically poured into the mediastinum by the surgeon. To ensure blindness, 100 cc of normal saline was further poured into the mediastinum by the surgeon and scrub nurse in the other two groups. In all three groups, the study solutions were prepared beforehand by the anesthesiologist in charge of the project and poured into the scrub nurse's gallipot to be poured into the patient's mediastinum.
Therefore, the surgeon and scrub nurse were unaware and blind to the type of solutions poured into the patient's mediastinum. After pouring the solutions into the patient's mediastinum and if conditions were stable and hemodynamics were maintained, patients were weaned off the heart-lung pump.
After this process and the injection of protamine and ensuring the patient's hemodynamic stability, for group C or IV, 1 g of tranexamic acid (equivalent to 10 cc) was injected IV. The same volume of 10 cc normal saline was injected into patients in the other two groups (oral and local). The drugs mentioned in all three groups were previously prepared by the anesthesiologist in charge of the project and provided to the anesthesia technician working on the project. The syringes were only labeled with A, B, and C, and also the technician was unaware and blind to the groups. Therefore, only the anesthesiologist who had previously prepared both IV and local drugs and provided them to the scrub technician was aware of the study groups. Patients were admitted 3 to 4 days before surgery to the CCU or surgical ward, and all patients' clopidogrel was discontinued. Aspirin consumption continued until the day of surgery. All study patients were operated on by a single surgeon to ensure the surgical procedure and type of surgery were identical for all patients, eliminating any potential confounding effects in this study.
Statistical analysis
The aforementioned questionnaires were statistically analyzed using the SPSS-19 software, and the data were presented in tables and statistical charts. Chi-square tests, One-way analysis of variance (ANOVA), or its non-parametric equivalent were used for data analysis.
Ethical considerations
In this study, patients' names and details were recorded confidentially, and no costs were imposed on the patients' families. Patients' information collection forms were completed with their written consent, and information about tranexamic acid, its uses, and potential side effects were provided to the patients and their families.
In all stages of the research, the investigators were obligated to comply with the ethics guidelines approved by the Ministry of Health and the Helsinki Declaration. This research project has been approved by the Ethics Committee of the Arak University of Medical Sciences with no. 6336 and ethics code IR.ARAKMU.REC.1400.114 and IRCT code IRCT20141209020258N166.
Results and Discussion
Characteristics of patients
A total of 96 patients, comprising 63 males and 33 females, were included in the study, with a mean age of 64.2 ± 6.8 years (Table 1). No significant differences were observed among the three groups concerning age and sex (P-value > 0.05). The comparison of the number of grafts in patients eligible for coronary artery bypass surgery revealed that the mean number of grafts in the injectable tranexamic acid group (3.9 ± 0.98), the oral tranexamic acid group (4.1 ± 1.8), and the local tranexamic acid group (3.6 ± 1.1) did not differ significantly (P-value = 0.421). Likewise, the average surgery time in the three groups was 5.9 ± 1.1 hours in the injectable group, 6.1 ± 2.1 hours in the oral group, and 6.4 ± 1.8 hours in the local group (P-value = 0.624). The average cardiopulmonary bypass time was 123.9 ± 10.4 minutes in the injectable group, 119.8 ± 9.8 minutes in the oral group, and 121.4 ± 8.8 minutes in the local group (P-value = 0.411). Furthermore, the average aortic cross-clamp time was 69.2 ± 5.6 minutes in the injectable group, 67.3 ± 4.8 minutes in the oral group, and 68.3 ± 4.9 minutes in the local group (P-value = 0.423).
Based on the results obtained, no significant difference was observed in the length of surgery time, average cardiopulmonary bypass time, and average aortic cross-clamp time among the three study groups.
Outcomes
The results of the bleeding score showed that the average bleeding score in the injectable tranexamic acid group was 3.9±0.88, in the oral tranexamic acid group was 4.1±1.1, and in the local tranexamic acid group was 4.4±0.98. However, since the p-value was 0.221, the difference was not statistically significant. Similarly, no significant difference was observed in terms of the average number of packed red blood cells received during and after surgery among the three groups. The average number of packed red blood cells received by patients in all three groups was approximately the same, with each group receiving an average of 2 units. In addition, the average number of platelets and fresh frozen plasma received did not differ significantly among the three groups (Table 2).
In terms of postoperative outcomes, the comparison of the return rate to the operating room and the occurrence of mortality revealed no significant difference among the three groups. The percentage of patients returning to the operating room in all three groups was 3.13% (P-value > 0.05). The occurrence of tamponade was also similar in the three groups, with each group having a rate of 3.13% (P-value > 0.05) (Figure 1).
According to Table 3, no significant difference was observed among the three groups in terms of the length of stay in the Intensive Care Unit (ICU) and the hospital. The bleeding score, the amount of packed red blood cells, platelets, and fresh frozen plasma received, were also approximately the same in each of the three study groups, with no noticeable differences (Figure 2).
Table 1: Comparison of age and sex in patients candidates for CABG surgery
|
Intravenous Tranexamic Acid Group |
Oral Tranexamic Acid Group |
Topical Tranexamic Acid Group |
|
|
Mean Age (years) |
64.3±6.4 |
64.6±5.2 |
63.9±8.4 |
|
Male |
22 (66.6%) |
21 (65.4%) |
20 (64.6%) |
|
Female |
10 (33.4%) |
11 (34.6%) |
12 (35.4%) |
Table 2: Comparison of mean received blood products in patients candidates for CABG surgery
|
Intravenous Tranexamic Acid Group |
Oral Tranexamic Acid Group |
Topical Tranexamic Acid Group |
P-value |
|
|
Mean Packed Red Blood Cells |
1.9±0.78 |
2.1±0.98 |
2.4±0.85 |
0.422 |
|
Mean Platelets |
3.1±0.95 |
3.5±0.89 |
3.8±0.87 |
0.402 |
|
Mean Fresh Frozen Plasma (FFP) |
2.2±0.78 |
2.8±0.98 |
2.3±0.86 |
0.412 |

Figure 1: Comparison of the percentage of reoperation and mortality rate in candidates for CABG surgery
Table 3: Comparison of mean and standard deviation of ICU and hospital length of stay in patients candidates for CABG surgery
|
Intravenous Tranexamic Acid Group |
Oral Tranexamic Acid Group |
Topical Tranexamic Acid Group |
P-value |
|
|
Mean ICU Length of Stay (days) |
5.8±1.1 |
5.2±1.2 |
5.1±1.4 |
0.630 |
|
Mean Hospital Length of Stay (days) |
10.6±1.4 |
11.1±1.9 |
10.9±2.1 |
0.422 |

Figure 2: Comparison of bleeding score, received pack cells, platelets, and plasma in candidates for CABG surgery pre- and post-operation
Overall, the study did not find any statistically significant differences in bleeding scores, blood product usage, return to the operating room, mortality, tamponade occurrence, and length of stay among the patients who received intravenous, oral, and topical tranexamic acid in the context of coronary artery bypass grafting surgery. Finding an optimal combination to prevent and reduce bleeding after surgery in patients who are candidates for coronary artery bypass grafting (CABG) is one of the goals of anesthesiologists, cardiologists, and heart surgeons. In our study of 96 patients who were candidates for CABG, patients were divided into three equal groups receiving medication orally, injectable, and topically. The results obtained from the study of these three groups indicate that the use of tranexamic acid in three forms- oral, injectable, and topical- is all effective in reducing postoperative bleeding and the frequency of returning to the operating room. The results of this study showed no significant difference among the three groups in terms of the amount of bleeding within 72 hours after the operation. In other words, tranexamic acid used in three ways (oral, injectable, and topical) was all effective in reducing postoperative bleeding and had a similar impact on this outcome. In terms of the number of times patients returned to the operating room, those receiving tranexamic acid returned less frequently than those who did not. However, the average frequency of returning to the operating room for patients who were candidates for CABG was the same in all three groups receiving tranexamic acid, suggesting that the three ways of using this drug had a roughly similar effect. There was no significant difference in the reception of blood products among the three groups, with average received pack cell, platelet, and FFP being the same across all groups. The results of this study are in line with previous studies. A 2013 study by Aoki M. et al. in Japan found that the effect of topical tranexamic acid on aspirin-induced bleeding in CABG was entirely evident and significantly reduced postoperative bleeding in these patients [16]. This is in line with our study because topical tranexamic acid was effective in reducing postoperative bleeding and did not differ significantly from the oral and injectable forms of the drug. In a 2017 study conducted by Prakash Jatin et al. in India, patients were divided into three groups receiving the drug injectably, topically, and a placebo. The results showed that in all three groups, postoperative bleeding was less than in the control (placebo) group. Also, the receipt of blood products in these three groups was less than in the control group. On the other hand, the use of topical tranexamic acid was as effective as injectable tranexamic acid in reducing postoperative bleeding [17]. The results of this study fully coincide with ours because in this study, the use of tranexamic acid orally, injectable, and topically was effective in reducing postoperative bleeding, and the effect of tranexamic acid in all three groups was the same and did not significantly differ from each other.
In a study conducted by Fawzy H. et al. in Canada in 2009 on patients undergoing CABG, the effect of topical tranexamic acid on reducing postoperative bleeding was investigated. The results of this study showed a significant reduction in postoperative bleeding when tranexamic acid was topically applied to the pericardial space, without an increase in postoperative complications in these patients [18]. These results are consistent with our study, as we also found that tranexamic acid, in all three forms (topical, oral, and injectable), was effective in reducing postoperative bleeding, and there was no significant difference in the effect of the drug among the three groups. In a study conducted by Hosseini et al. in Iran in 2014, the effect of topical tranexamic acid was examined in patients undergoing CABG. In this randomized controlled trial, patients were divided into two groups, one receiving topical tranexamic acid in the mediastinal and pericardial spaces and the other serving as a control. The results of this study showed that topical tranexamic acid led to a reduction in postoperative bleeding in these patients [19], which is consistent with our study.
In a study conducted by Habbab M. et al. in Canada in 2019, the effectiveness of topical tranexamic acid was compared with the injectable form of the drug in patients undergoing open heart surgery. Patients were divided into two groups, one receiving topical tranexamic acid and the other receiving the injectable form, and the amount of bleeding, blood product usage, and complications were compared. The results of this study showed that topical administration had a similar effect to the injectable form in reducing bleeding, decreasing blood product usage, and reducing complications associated with injectable tranexamic acid [20]. These results are also in line with our study. In this study, both topical and injectable forms of the drug were effective in reducing postoperative bleeding and blood product usage, and there was no significant difference in the efficacy between the different forms of the drug. In a study conducted by Ozturk A. et al. in Turkey in 2021, the efficacy of topical and injectable forms of tranexamic acid was examined in knee joint replacement surgery. The results of this study showed that both the injectable and topical forms of the drug were effective in reducing bleeding and blood product usage, and there was no significant difference between the two methods of drug administration [20]. These results are consistent with our study, as we also observed a reduction in postoperative bleeding and blood product usage in all three groups receiving the drug, and there was no significant difference in the efficacy of tranexamic acid among the three groups. Comparing the results of our study with previous studies indicates the effectiveness of all three forms - oral, injectable, and topical - of tranexamic acid in reducing postoperative bleeding, the number of reoperations, and blood product usage. The lack of significant difference among the three groups in terms of outcomes suggests a similar effect of different methods of tranexamic acid administration in reducing postoperative bleeding. The results of this study clearly demonstrate that topical administration of tranexamic acid can be as effective as the injectable and oral forms in reducing postoperative bleeding and the number of reoperations, while the adverse effects associated with topical tranexamic acid are much less than the injectable and even oral routes.
Conclusion
The use of tranexamic acid in all three forms (oral, injectable, and topical) has proven to be effective in reducing postoperative bleeding, and this effect is consistent across all three groups. Furthermore, the impact of tranexamic acid in its oral, injectable, and topical formulations on reducing the frequency of reoperation and blood product utilization is comparable.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
ORCID
Behnam Mahmodiyeh
https://orcid.org/0000-0002-5554-6866
Alireza Rostami
https://orcid.org/0000-0002-0445-729X
Ava Akhavan
https://orcid.org/0000-0003-1986-7942
Amir Almasi
https://orcid.org/0000-0003-4434-561X
HOW TO CITE THIS ARTICLE
Behnam Mahmodiyeh, Alireza Rostami, Ava Akhavan, Amir Almasi, Alireza Kamali. Comparative Analysis of the Effect of Intravenous, Topical, and Oral Tranexamic Acid on Reducing Bleeding After Coronary Artery Bypass Surgery. J. Med. Chem. Sci., 2024, 7(2) 426-435.