Document Type : Original Article
Authors
- Helmia Farida 1, 2
- Loraine Harinda 2
- Hanna Fraleya Pattipeiluhu 3
- Bazilah Dayana 4
- Mujahidah Mujahidah 5
1 Faculty of Medicine, Diponegoro University, Department of Microbiology, Semarang, 50275, Indonesia
2 Faculty of Medicine, Diponegoro University, Department of Pediatrics, Semarang, 50275, Indonesia
3 Ridwan Meuraksa Hospital, Department of Microbiology, Jakarta, 10430, Indonesia
4 Medical Profession Study Program, Faculty of Medicine, Diponegoro University, Semarang, 50275, Indonesia
5 Dr. Kariadi Hospital, Department of Microbiology, Semarang, 50244, Indonesia
Abstract
Objectives: The third-generation cephalosporins have been very widely used in Indonesia, raising concerns about the increase of infections caused by multidrug resistance organisms, in particular the extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-pE). Dr. Kariadi Hospital (DKH), Semarang, Indonesia, implemented the policy of ceftriaxone restriction since November 2018. This study aimed to describe the prevalence of urinary tract infections (UTI) caused by ESBL-pE during 2018 -2019, the origin of the ESBL-pE strains, the risk factors of developing ESBL-pE UTI, and the antibiotic options for ESBL-pE UTI paediatric patients.
Methods. A retrospective cross-sectional study was performed by collecting data from microbiology laboratory and medical records of paediatric patients hospitalized with UTI in DKH, from January 2018 to December 2019. Statistical analysis was performed using Chi-square or Fisher-exact test.
Results: UTI was microbiologically confirmed in 318. Enterobacteriacea caused UTI in 86 (59%) patients, of these 56.4% (in 2018) dan 62.9 (in 2019) were ESBL-pE strains. E. coli and K. pneumoniae were the most common etiology of UTI. ESBL-pE was isolated from 42% UTI patients from the community and from 76% UTI patients referred from other hospitals (p <0.01). Most strains were susceptible to amikacin, meropenem, and fosfomycin with a tendency of lower susceptibility in 2019 than in 2018 (p>0.05)
Conclusion: The UTI incidence caused by ESBL-pE in DKH was high. Patients referred from other hospitals were important source of ESBL-pE UTI, underlining the urgency to perform surveillance and to implement the infection control and prevention measures in addition to the antibiotic stewardship program.
Graphical Abstract
Keywords
Introduction
The third-generation cephalosporins have been very widely used in Indonesia due to their cheap price, broad-spectrum, and clinical efficacy in the past few years [1]. This situation raises concerns about the emergence of multidrug-resistant organisms, in particular the extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-pE) in Indonesian hospitals as many studies reported that use of the third generation cephalosporines as a risk factor for the increase of ESBL-pE prevalence [2, 3]. The Indonesia National Committee on Antimicrobial Resistance (KPRA) reported that the prevalence of blood stream infections caused by ESBL-pE in Indonesian hospitals increased from 40.0% on average in 2013 to 62.2% in 2016 [1].
Urinary tract infection (UTI) is one of the most common infections in children, which can develop to kidney damage and septicaemia. Most UTI are caused by the Enterobacteriaceae family, with Escherichia coli and Klebsiella pneumoniae occupying the highest rank among other species of Enterobacteriaceae [4, 5]. Therefore, we need to be aware of increasing incidence of UTI caused by ESBL-pE in paediatric patients. ESBL is an enzyme that hydrolyzes most β-lactam antibiotics including penicillin, the first, the second, and the third-generations of cephalosporins, and aztreonam groups. This enzyme is produced by mutated β-lactamase genes which is easily transmittable by many means within a hospital and between hospitals [6, 7]. UTIs caused by ESBL-pE are more difficult to treat, leading to the increase of the length of stay, treatment failure, costs, complications, and in turn, increasing risk for the surrounding community to getting an infection by multidrug-resistant organisms, as well as the cost burden for finding new and more potent antibiotics [8].
Dr. Kariadi Hospital (DKH) is a tertiary referral hospital in Semarang City, Indonesia, of which the prevalence of bacteremia cause by ESBL-pE reached 72% in 2016 [1]. In November 2018 the director of DKH decided to implement a ceftriaxone restriction policy to control and reduce the infections prevalence caused by ESBL-pE, i.e. ceftriaxone is only used for infections in central nervous system, severe leptospirosis, sepsis, and typhoid in pregnant patients.
This program was effectively commenced in January 2019. By the end of 2019, overall ESBL-pE prevalence decreased from 71.7% to 55.5%, as described from patients’ blood, urine, and sputum cultures (data are not published). However, there are no specific data from the Paediatric ward. Therefore, this study aimed to describe the UTI incidence caused by ESBL-pE among paediatric patients hospitalized in DKH, to identify the origin of patients, and to explore the antibiotic choice for pediatric patients with UTI caused by ESBL-pE in 2018 and 2019.
Materials and Methods
The study retrospectively included paediatric patients aged 1 month to 17 years old hospitalized with UTI between January 2018 and December 2019. Data related to the patients were taken from medical records. Data on pathogens and antibiograms were taken from Microbiology Laboratory. The identification, ESBL-p phenotypic confirmatory and antimicrobial susceptibility tests were performed with VITEK®2 Compact (bioMérieux, Marcy l'Etoile, France), except for fosfomycin and cefoperazone-sulbactam of which the susceptibility tests were performed using disc diffusion method (Oxoid, Basingstoke, UK). The UTI diagnosis was confirmed if patients developed symptoms and signs of UTI (fever > 38.0 oC or hypothermia < 36.0 oC, dysuria, frequency, suprapubic pain, and urgency), leukocyturia of > 5/ HPF or leukocyte esterase positive, or nitrite positive AND bacterial count was >105/mL for urine collected by clean-catch mid-stream or >104/mL for urine collected from a urinary catheter or from a clean-catch mid-stream of a patient who had taken antibiotic compatible with the susceptibility profile. Patients with positive urine cultures of non-uropathogens were excluded. Chi-square or Exact-Fischer was used for statistical analysis where appropriate.
Results and Discussion
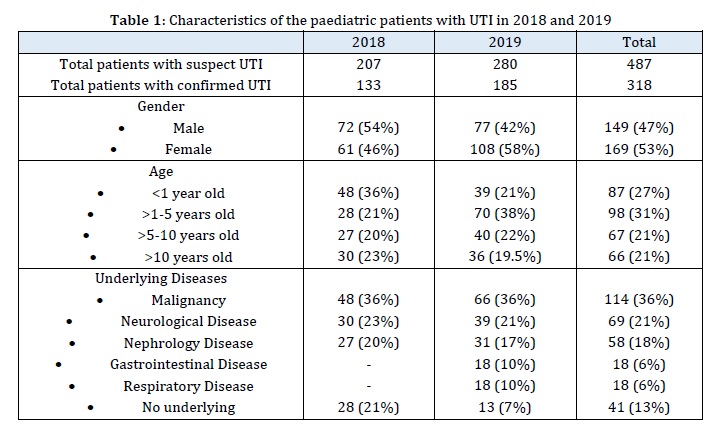
In total, 487 paediatric patients were suspected of developing UTIs and urine cultures were performed. UTIs were confirmed in 318 (65%) patients. Subjects' characteristics are presented in Table 1. Most (58%) of patients aged < 5 years old, with malignancy, neurology, and nephrology problems as the dominant underlying diseases.

The uropathogen species are listed in Table 2. In total, Enterobacteriaceae was isolated in almost 60% of the UTI patients, with E. coli as the most frequent etiology of UTI (30-35.7%), followed by Klebsiella sp. (18.4-19.5%). The ESBL-pE prevalence out of the Enterobacteriaceae was 56.4% in 2018 and 62.9% in 2019 (p = 0.14), or 32.3% out of all positive urine cultures in 2018 and 36.8% in 2019 (p = 0.36).
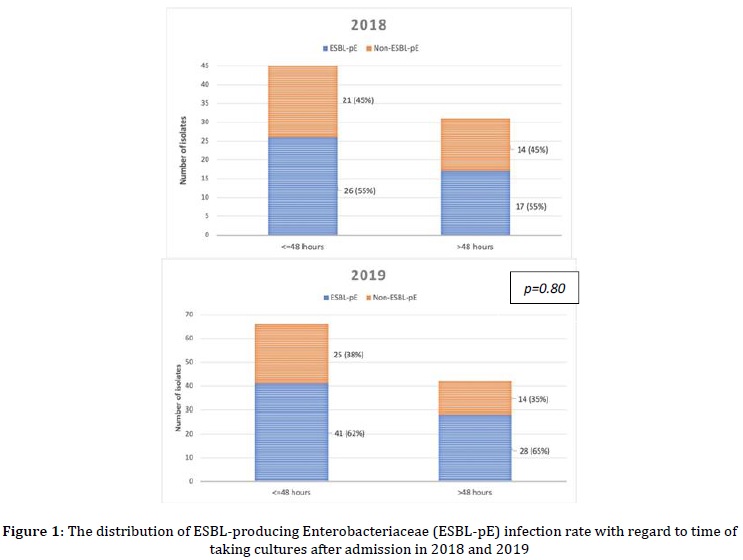
To reveal whether the UTI caused by ESBL-pE strains developed before or after hospital admission, the ESBL-pE isolates were compared with regards to the time of taking urine culture (Figure 1). In 2018 and 2019, on average, 60% of urine cultures were taken before-48 hours of hospitalization. Out of the cultures taken before-48 hours, 55% (in 2018) and 62% (in 2019) were ESBL-pE strains, implying that most of UTI caused by ESBL-pE had developed before the patients were admitted to the hospital and were not acquired in DKH.
As DKH is a third-level referral hospital, we also explored whether the ESBL-pE were originated from community or from other hospitals, by comparing the prevalence of ESBL-pE UTIs Enterobacteriaceae with regards to patients’ origin, as depicted in Figure 2. This figure shows that in both 2018 and 2019, most UTIs in patients referred from other hospitals were caused by ESBL-pE strains (78% and 73%, respectively). Out of the patients originated from the community, non-ESBL-pE strains were still a more dominant cause of the UTIs. However, there was a quite remarkable increase in the percentage of community-acquired ESBL-pE UTI from 2018 to 2019 (36% vs 48%) although not statistically significant (p = 0.3).
The susceptibility pattern of ESBL-pE strains is demonstrated in Figure 3. Most strains were still susceptible to amikacin, doripenem, fosfomycin, meropenem, and cefoperazone-sulbactam. However, although not statistically significant, the percentage of susceptibility to most antibiotics had decreased from 2018 to 2019, for instance, amikacin (from 100% to 93%), and meropenem (from 96% to 92%).


The susceptibility to amoxicillin-clavulanat, ampicillin sulbactam, and cefepim was very low (0%, 2%, and 4% on average, respectively), while that to piperacillin-tazobactam, ciprofloxacin, and gentamicicn was moderately low (67%, 62%, 57% on average, respectively).
The risk factors for getting ESBL-pE infections were in general similar between 2018 and 2019. Re-hospitalization within the last one year was found as a risk factor (OR 3.0; 95% CI: 1.31-6.82; p=0.010), as well as urinary catheterization (OR 2.70 (95%CI: 1.20-6.05; p=0.016)). History of surgical procedures did not increase the risk of ESBL-pE infection (OR 2.0; 95%CI: 0.89-4.392; p=0.096).
This study confirmed a very high prevalence of ESBL-pE UTI in this hospital (56,4% in 2018 and 62.9% in 2019) compared to other hospitals in other countries which ranged from 9% to 42% [9-12]. This condition is not very surprising because the antibiotic use in Indonesia is high, with a higher proportion of antibiotic use was without indication [13-15], and the third-generation cephalosporines were the most frequently prescribed in most Indonesian hospitals [16]. Therefore, it is very likely that the very high prevalence of ESBL-pE UTI in paediatric patients also develops in other hospitals in Indonesia.
The implementation of ceftriaxone restriction policy in DKH from November 2018 has decreased the overall prevalence of infections caused by ESBL-pE at the hospital level of DKH from 71.7% to 55.5% (data not published). However, in the Paediatric ward, specifically for UTIs, the prevalence of ESBL-pE Enterobacteriaceae increased from 56.4% to 62.9% in 2019 although not statistically significant. This could be caused by the high proportion (more than 75%) of the patients with UTI referred from other hospitals had already developed ESBL-pE UTI prior to the admission to DKH. As far as our observation, these referring hospitals did not implement an antibiotic stewardship program; even ceftriaxone had been becoming the most frequently used antibiotic there. Consequently, it could be estimated that the ESBL-pE prevalence in the referring hospitals are high or even higher than in DKH. The referred patients may directly or indirectly spread ESBL-pE they acquired from the referring hospital to other patients in DKH, causing causing the ESBL-pE prevalence in the Paediatric ward of DKH to be remain high despite the ceftriaxone restriction policy. Infection prevention and control measures such as contact isolation and hygiene practice are thus important to be established in this situation. Ideally, referred patients from other hospitals are screened and isolated until proven that they are not colonized or infected by ESBL-pE. However, this strategy would probably be very difficult to apply in Indonesian setting as there is no specific fund for this means.
As a third level referral hospital, DKH cares many patients with malignancy, immunodeficiency, and other underlying diseases which may affect immune system, and then increase the length of stay and the risk of getting nosocomial infections including UTI. As presented in Table 1, the proportion of UTI patients with underlying disease(s) in 2019 (93%) was much higher than in 2018 (79%); this may also contribute to the higher prevalence of ESBL UTI in 2019. Another possible reason for the increase of ESBL-pE UTI prevalence in 2019 is the quantity of ceftriaxone and other third-generation cephalosporines used at the Paediatric ward was probably still high in 2019 despite the restriction policy. This could be influenced by the number of patients to whom treatment with ceftriaxone was still permitted according the DKH’s ceftriaxone restriction policy, as well as the doctors’ compliance to get rid of ceftriaxone in treating other diseases. We do not have the data on the quantity of all the third generation cephalosporine use and number of patients with diseases permitted to be treated with ceftriaxone in the Paediatric ward of DKH to find out why the prevalence of ESBL-pE UTI in the Paediatric ward did not decrease while the overall prevalence of ESBL-pE at hospital level has already decreased after the implementation of ceftriaxone restriction policy. The timing of observation after the implementation of the ceftriaxone restriction policy in this study, i.e. within one year, may also too short to result in the decrease of such prevalence. A study in Denmark reported that the prevalence of ESBL producing K. pneumoniae infection started to decrease after two years of the implementation of the third generation cephalosporine restriction combined with other multidisciplinary programs [17]. A study in France showed that antibiotic restriction alone had lowest impact in reducing the prevalence of ESBL infections as compared to hand hygiene and cohorting [18].
This study also documented that among patients admitted from the community (thus, not referred from other hospitals), the ESBL-pE UTI prevalence was also high (36% in 2018 and 48% in 2019), raising the question whether the ESBL-pE strains have been widely distributed in the community. Several studies reported a high carriage of ESBL-pE in the community of developing countries [19, 20]. The same condition may probably exist in Indonesia [21] which underlines the urgency to study and control antibiotic use in farming and agriculture industries as a potential reservoir for ESBL-pE and other multidrug-resistant organisms in the community [22]. Studies on the use of the oral third-generation cephalosporines and the carriage of ESBL-pE in the community, are also very important to do.
In addition, our study showed that all ESBL-pE isolated from paediatric patients with UTI in Dr. Kariadi hospital still were susceptible to amikacin. Therefore, this antibiotic can be used as the first-line antibiotic for UTI caused by ESBL-pE, particularly in patients with no complication, provided that amikacin is highly concentrated in urine and less broad-spectrum [23, 24]. However, there is a tendency that amikacin susceptibility to decrease in this hospital only after one year from 100% to 93%. Nitrofurantoin is further a drug of choice for ESBL-pE UTI because this antibiotic works only in urinary tract, thus minimize systemic selective pressure which lead to the emergence of resistance [25]. Unfortunately, this drug is not available in Indonesia.
This study has some limitations due its retrospective design and reliance on medical records, some of the data were not available for all the patients. In addition, this study could not define ESBL-pE UTI which acquired during hospitalization in DKH itself, as urine cultures taken after 48 hours did not always reflect that the UTIs were developed in DKH hospital. Instead, this could be because the doctor postponed taking urine cultures for any reasons.
This study underlines the importance of implementing infection prevention measures along with antimicrobial stewardship program as “one unseparated package”. When a clinician cares a patient infected (or colonized) by multidrug resistant organisms (MDRO) such as an ESBL-pE, he or she not only needs to give proper antibiotic(s) to the patient, but also prevent the MDRO from transmitting to other patients or to hospital environment. Otherwise, the MDRO may infect many other patients [26].
Controlling antimicrobial resistance in hospitals is a complicated task involving many parties at hospital level and national levels. The Ministry of Health (MoH) is responsible to develop and coordinate a systematic comprehensive national program for antimicrobial resistance control in the national health systems, as well as collaborating with other parties, such as the ministry of farming and animal husbandry, the ministry of finance, and the health insurance providers. The MOH needs to develop an appropriate national drug formulary that can minimize the use of broad-spectrum antibiotics such as the third-generation cephalosporines. Unfortunately, current Indonesia national drug formulary even stimulates the use of broad-spectrum antibiotics which escalate the resistance problems because it includes very few broad-spectrum antibiotics. For instance, narrow spectrum antibiotic such as dicloxacillin or flucloxacillin are not included in the formulary. Moreover, the formulary does not include specific urinary tract acting antibiotics such as nitrofurantoin. Most antibiotics included in the national formulary are broad spectrum. On the other hand, the number of capable microbiology laboratory is limited. Most hospitals in Indonesia do not have a microbiology laboratory. Inevitably, doctors tend to give empirical therapy with broad-spectrum and inexpensive antibiotics such as the third-generation cephalosporins.
Although it is hard, a sustainable comprehensive measure in controlling antimicrobial resistance is worth fighting for, since the very high prevalence of ESBL-pE infections increase the use of carbapenems, which in turn, will increase the prevalence of carbapenem resistant organisms which will have a very serious impact on health services in Indonesia [27]. The Indonesia government should consistently follow and implement the World Health Organization (WHO) recommendation to prevent and control antibiotic resistance in Indonesia.
Conclusion
The incidence of urinary tract infection caused by ESBL-pE in this hospital was very high despite the implementation of ceftriaxone restriction policy within one year. The patients referred from other hospital are important source ESBL-pE. Amikacin can be used as the first choice of antibiotic for UTI caused by ESBL-pE to prevent the emergence of carbapenem resistance problems. A proper infection control along with antibiotic stewardship programs together are urgent and essential to be implemented at the national and hospital level.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Orcid
Helmia Farida
https://orcid.org/0000-0002-3493-9847
Loraine Harinda
https://orcid.org/0009-0008-5357-1399
Hanna Fraleya Pattipeiluhu
https://orcid.org/0009-0004-8547-9344
Bazilah Dayana
https://orcid.org/0009-0001-4116-9019
Mujahidah Mujahidah
https://orcid.org/0009-0004-0831-7325
HOW TO CITE THIS ARTICLE
Helmia Farida, Loraine Harinda, Hanna Fraleya Pattipeiluhu, Bazilah Dayana, Mujahidah Mujahidah. Urinary Tract Infection Caused by the Extended-Spectrum β-lactamase (ESBL)-Producing Enterobacteriaceae in Hospitalized Pediatric Patients before and after Ceftriaxone Restriction Policy in a Tertiary Referral Hospital. J. Med. Chem. Sci., 2023, 6(10) 2357-2366


