Document Type : Original Article
Authors
Department of Pharmacology, SRMIST, Kattankulatthur, Tamil Nadu, 603203, India
Abstract
Females of childbearing age often suffer from polycystic ovary syndrome (PCOS), a hormonal condition. Symptoms including insulin resistance, high androgen levels, irregular menstrual (estrous) cycles, and polycystic ovaries are linked to this condition. The study's objective is to determine the therapeutic effect of Tinospora cordifolia stemextract and Alkaloid Rich Fraction against continuously Light Induced Polycystic Ovary Syndrome in Sprague Dawley (SD) female rats. Animals were grouped into six groups where the control group received only distilled water and the standard group received Clomiphene citrate 20 mg/Kg. The test groups received Tinospora cordifolia Stem Extract (200 mg/kg b.w and 400 mg/kg b.w) and Alkaloid Rich Fraction (10 mg/kg b.w and 20 mg/kg b.w) for the period of 4 weeks after PCOS induction. The results showed a significant reduction in elevated testosterone levels compared to that of the PCOS group (Light Induced Group (L/L 24 hrs Continuous), but follicle-stimulating hormone (FSH), insulin levels reported significant increase compared to that of the L/L PCOS group (Light Induced Group (L/L 24 hrs Continuous) and increased expression of INSR gene and restoration of melatonin levels. From the results, it was concluded that High doses of Tinospora cordifolia Stem Extract (400 mg/kg b.w) and Alkaloid Rich Fraction (20 mg/kg b.w) could be effective against Continuously Light Induced Polycystic Ovary Syndrome in Rodents it has due to decreased testosterone levels and increase insulin sensitivity by up-regulating INSR gene expression followed by restoration of melatonin levels. After treatment period a significant decrease in elevated LH levels, testosterone levels, and a significant increase in insulin and follicle-stimulating hormone (FSH) levels when compared to the L/L PCOS group (Light Induced Group (L/L 24 hrs Continuous), as well as increased INSR gene expression and restored melatonin levels.
Graphical Abstract
Keywords
Main Subjects
Introduction
Light plays an important role in our daily life. In the follicular phase of the menstrual cycle, exposure to light in the morning encourages the release of hypophyseal reproductive hormones. However, the effects of continuous/prolonged light exposure on female rats induces hyperandrogenism [1]. In contemporary life, voluntary sleep restriction is becoming widespread. Disturbances in circadian rhythm is a critical risk element for PCOS, Obstructive sleep apnea (OSA) is more likely in women with PCOS and sleep disruption is one aspect that is frequently disregarded [2].
Circadian rhythm disruption has recently drawn a lot of attention among the environmental factors that might cause PCOS. As a classic illustration, shift employment causes a severe circadian misalignment due to the light exposure and altered eating and sleeping schedules [3]. Numerous investations have been demonstrated that women who worked night shifts experienced irregular periods, which were usually linked to dysmenorrhea; deregulated glucose metabolism, metabolic syndrome and insulin resistance are other known risk factors for PCOS [4]. Melatonin plays an important role in coordinating our sleep-wake cycle with day and night by regulating circadian rhythm, and appears to protect follicles from reactive oxygen species. In addition, it regulates folliculogenesis, ovarian steroidogenesis, and oocyte maturation [5]. Disturbances in circadian rhythm are associated with an increase in obesity, metabolic illness, and insulin resistance. Insulin resistance is a key feature of both obesity and PCOS. Therefore, it is important to investigate the potential connection between PCOS, night shift work, and circadian disturbance. It is generally known that melatonin production is reduced after being exposed to light, and various studies have linked the melatonin suppression at night to an increased risk of diabetes and insulin resistance [6, 7].
Cyclical light-dark photoperiods regulate the LH surges that cause ovulation in rodents. These light-dark photoperiods can be disturbed within a timeframe of 24 hours, which can interfere with rat reproductive cycle and limit ovulation [8]. As a substitute method for causing PCOS, exposing female rats to a continuous light environment has been proposed [9]. According to the recent studies and literature survey, rodents can develop PCOS in less than 75 days when exposed continuously to 600-lux light [10].
The involvement of circadian rhythm disruption in the PCOS pathogenesis has not yet been fully addressed, despite several investigations uncovering possible explanations. In this current research on Sprague Dawley (SD) rats, we continuously exposed them to light for 16 weeks while observing their reproductive and metabolic effects.
Tinospora cordifolia, stems are rich in isoquinoline alkaloids [11], and it is utilized in Ayurveda for its stress-relieving and vigor-improving rasayana benefits. In the Ayurveda treatment regimen conducted on sub-fertile women aged 25 to 40 years,T. cordifolia is one of the plants in treatment regimen were taken 5 gms twice daily, but not scientifically proved [12]. T. cordifolia have numerous medical benefits, including anti-oxidant, adaptogenic, anti-inflammatory, anti-estrogenic, and immunomodulatory, in endocrine disorders, anti-diabetic, hypolipidaemic, etc. have been proven. This study's objective was to ascertain the effect of T. cordifolia stem extract and alkaloid rich fraction against insulin gene INSR expression, estimation of melatonin by HPLC method, serological evaluation of hormonal levels, histopathological examination of ovarian, and other tissues upon constant light exposure-induced PCOS rodent model, possible link between PCOS, working the night shift, and circadian disruption.
Materials and Methods
Animals
Forty two female Sprague Dawley (SD) rats of 200-220 g weight approximately 6 weeks were procured from Suppliers and Breeders of Experimental Animals Venkateshwara Breeders and Laboratory animals; No. 4304, 13th Mn, 1st Cross, Subramanya Nagar, Bangalore, Karnataka – 560021. The Institutional Animal Ethical Committee's recommendations and CPCSEA guidelines were followed in all animal procedures, SRM College of Pharmacy, SRMIST, Kattankulathur. IAEC number; IAEC 241/2021. All the animals were housed in identical polypropylene cages at a constant temperature of 22 °C and 55-65% humidity. Before the trials, rats were acclimatized for a week, while having free access to standard chow and demineralized water. Throughout the experiment, rats were allowed freely for food and water.
Materials and equipment design
The custom-made light experimentation box was 180 cm in height, 45 cm in width, and 120 cm in length. It was divided vertically into four identical, distinct compartments with measurements of 120 cm, 45 cm, and 45 cm apiece. Each chamber had its own thermocol sheet-made ventilation system. Each chamber was equipped with a fluorescent lamp (colour temperature: 6500 K, illumination: 600 lux), whose lights were monitored and checked for free modification of the illumination. A microscope was used in this investigation (stanchem tn655, India), the required chemicals, solvents, and reagents were obtained from Sigma-Aldrich.
Continuous light induction
All female rats in each chamber were put beneath the light experiment box and randomly divided into control and experimental/treatment groups. Rats in the experimental/treatment group underwent a 16-week period of continuous light exposure (lights turned on at 8 a.m. Indian standard time), whereas the control groups underwent a 12-hour/12-hour light/dark cycle. (L/L, lights on 24 hours a day) [13].
Plant materials
In Ramasamy Chetty Naatu Marunthu Kadai, Rasappa Chetty Street, Park Town, Chennai, 600003, India, dried stems of Tinospora cordifolia were collected. Dr. K.N.Sunil Kumar, Research Officer and HOD for the Department of Pharmacognosy, Central Council for Research in Siddha, Ministry of AYUSH, Government of India, taxonomically recognised and verified the plant material. Certificate specimen no. T19122001S.
Tinospora cordifolia stem extraction
At an Arul mill close to Arumbakam, Tinospora cordifolia died stems were acquired, authenticated, and ground/milled. The dried stem powder was defatted with petroleum ether at 60-80 °C for 2 days, and then extraction in a soxhlet using a solvent combination of methanol:acetone (70:30; 2000 mL × 6 cycles) at 40 °C till twenty-four hours. The residue was dried under low pressure using a rotary evaporator.
Alkaloid rich fraction
Tinospora cordifolia stem that had been dried and ground into a coarse powder weighing 940 g was defatted using petroleum ether at 60-80 °C for 2 days, and the dry material was extracted with hydroalcoholic solvent using the soxhlet at 65-70 °C for one week. The collected solvent was diluted with 100 mL solution of hydrochloric acid to acidify the semisolid hydroalcoholic extract (29.7 g). The filtrate was filtered using Whatman filter paper, and then washed with diluted HCl until the alkaloid test proved successful. Alkalinization of the filtrate and washings (pH 8.0) using 25% ammonia before being partitioned by chloroform (CHCl3-3×150 mL, 2×100 mL). After that the chloroform layer was condensed, the alkaloid-rich fraction was pale yellow as shown Table 1 and illustrated in Figure 1 [14].
Treatment
In this study, through continuous Light Exposure environment (L/L, lights on 24 hours every day) for 16 weeks, rats will induce PCOS (Hyperandrogenism). Animals were randomized into 7 groups each in 6 animals (n=6). Group 1- vehicle control (control); Group 2- Disease control- PCOS (Light Induced Group (L/L 24 hrs Continuous); Group 3- Standard (L/L group with PCOS + Clomiphene citrate 20 mg/Kg), Group 4- Treatment L/L group with PCOS + Low dose of T.cordifolia stem extract (200 mg/kg body weight), Group 5- Treatment L/L group with PCOS + High dose of T.cordifolia stem extract (400 mg/kg body weight), Group 6- Treatment L/L group with PCOS + Low dose of T.cordifolia alkaloid rich fraction (10 mg/kg body weight), Group 7- L/L group with PCOS + High dose of T.cordifolia alkaloid rich fraction (20 mg/kg body weight) with a treatment period of four weeks through oral route of administration.
Collection of vaginal smears
After the first week of induction, a vaginal swab was obtained daily to monitor estrous cycles. Following soaking a sterile cotton swab in 0.9% saline, smeared along the initial third wall of vagina. The cotton swab was withdrawn and spread on a glass slide in the same way. Crystal violet (HiMedia Laboratories Pvt. Ltd., LBS Marg, Mumbai, India) was used to stain smear slides and they were examined at 100X magnification. All of the materials were evaluated, and photomicrography was conducted under a microscope in a blinded way (Labomed, M. G. Road, Gurgaon, India).
Blood collection and serum hormonal nalysis
Following the treatment period, the animals were fasted overnight and anaesthetized with thiopentane sodium 20 mg/kg IP. Blood samples were collected through the retro orbital plexus into separate tubes for biochemical analysis and were centrifuged for serum/plasma separation using a high speed cooling centrifuge at 3000 rpm for 15 minutes at 4 °C. Biochemical factors such as Estradiol, FSH, LH, Insulin, and Testosterone were investigated in rats using an ELISA kit.
INSR Expression analysis by RT PCR
Materials required
DEPC, TRIZOL reagent, Tris, EDTA, EtBr, and Acetic acid were bought from Sigma, (USA), 1X PBS and agarose were from Himedia, India. 96 well plate and wash beaker were obtained from Tarson, India. The forward and reverse primers were purchased from GCC Biotech PvtLtd., India. The 1st Stand cDNA synthesis kit was purchased from Roche (Switzerland). EGTA, Sodium acetate and Taq Mix (2X), BioLit, were purchased from SRL, India.
Primers used
Genes Directions Sequence (5´ – 3´)
INSR Forward 5′- GTCTCCTCGGATCAGAGCGC-3'
Reverse 5′- GAGTCCCTTCCTAGGCCAGATC-3'
β-actin Forward 5′- AGCCATGTACGTAGCCATCC -3'
Reverse 5′- CTCTCAGCTGTGGTGGTGAA -3'
The PCR amplification was carried out in a thermal cycler (PCR, Himedia).
RNA isolation
Following the manufacturer's instructions, the TRIZOL technique was used to isolate total RNA. To get the cell pellet, the tissue samples (M1, M2, M3, M4, M5, M6, and M7) were centrifuged at 5000 rpm for 10 minutes in DEPC-treated centrifuge tubes. 700 l of TRIZOL were added to the cell pellet (1×107 cells) to lyse the cells. After being aggressively pipetted into 1.5 mL tubes, the lysate was collected. 300 l of chloroform was added and vigorously stirred at room temperature for 5 min. Centrifugation was used to separate the aqueous layer for 20 min. at 40 °C at 12000 rpm. The 1.5 mL tube was used to capture the aqueous layer. 700 l of isopropanol were added to precipitate the RNA. Centrifugation was used to pellet the precipitated RNA for 20 minutes at 40 °C at 12000 rpm. 70% ethanol was used to wash the pellet. To prepare the air-dried RNA pellet for usage, 30 l of double-distilled autoclaved water was added. The Labman UV Vis Spectrometer was used to determine the quantity and quality of the extracted RNA.
DNAse treatment
The DNase process eliminated any DNA contamination that may have occurred during RNA production. The reaction volume, which included 1U of DNase, was adjusted to 20 l. After 30-45 minutes of incubation at 370 °C, 20 M of 2 l EGTA was added, and another 10-min incubation at 660 °C followed. Absolute ethanol (2V) and sodium acetate (1/10 V) were added, then the mixture was incubated at -200 °C for 60 min. The particle was then rinsed with 500 l of 75% ethanol and centrifuged at 12000 rpm for 20 min at 40C after that. The air-dried material was dissolved in 20 l of Milli-Q grade water, and then was put aside for subsequent use.
cDNA synthesis
Utilizing a reaction mixture comprising reverse transcriptase, 1.5 g of total RNA were transformed into cDNA (MMLV). The cDNA synthesis process took place at 25 ᵒC for 10 minutes and 420C for 59 minutes. Reverse transcriptase inactivation and denaturation of the cDNA/RNA hybrid were carried out at 99 ᵒC for 5 seconds, followed by a hold at 4 ᵒC.
Gene level expression detection
The target gene was amplified using the generated cDNA as a template. The Taq Mix (2X), BioLit was used to perform RT PCR [15] through PCR. RT-PCR was used to evaluate the particular genes' expression levels. Expression was normalized using the endogenous control (β-actin) was used as the calibrator.
PCR condition
Initial melting temperature for the INSR gene is 94 °C for 3 min, followed by 30 cycles of 94 °C for 30 sec. 30 sec of annealing at 59 °C, 1 min of extension at 72 °C, 10 min of final extension at 72 °C, and then a hold at 4 °C. Actin gene melting temperature: 94 °C for the first 3 min, and then 30 cycles of 94 °C for 30 sec. Annealing at 57 °C for 30 sec, followed by an extension at 72 °C for 1 min, a final extension at 72°C for 10 min, and a hold at 4 °C [16].
Agarose gel electrophoresis
A clear solution was created by boiling the (0.3 g) agarose powder in 30 mL of 1X TAE buffer. It was then allowed to cool, reaching a temperature of around 500 °C. Therafter, 1.5 L of ethidium bromide was added and well mixed. It was poured into a gel casting plate with a gel comb that had previously been adjusted, and it was left at room temperature for half an hour to solidify. In the electrophoresis tank, 1X TAE buffer was applied to the gel. Using micropipettes, 5 L of PCR product and 1 L of gel loading dye were added to the wells along with a 100 bp ladder. It was ran over 15 to 20 minutes at 70 V. The orange color (DNA) bands were observed in the UV gel documentation system. The bands were further analyzed using Image J software, USA.
Estimation of melatonin by HPLC method
Melatonin was purchased from Sigma-Aldrich (Calbiochem®, Darmstadt, Germany). Waters HPLC System (Vienna, Austria) was used to estimate serum melatonin levels. Detector: Array Detector (Waters G.M.B.H., Vienna, Austria) and Waters 2545 Quaternary Gradient Module with 2998 Photodiode. Column: SunFire (4.5×250 mm) C18 column, Software: The data were collected and processed with Empower 2.0 software.
Sample preparation
A sample of the chemical at a concentration of 1 mg/mL was collected and dissolved in HPLC-grade methanol. Next, for HPLC analysis, the compounds were filtered using a syringe filter (Whatman, GE Healthcare, UK, PVDF Filter Membrane with Polypropylene Housing Pore Size 0.2 m). Flow rate: 1 mL/min, Mobile phase: Methanol: Water (50:50), and Sample volume: 20 l. The took detection process conducted at 280 nm for 30 minutes. Each and every solvent was HPLC-grade and bought from Merck (Darmstadt, Germany).
Histopathological examination
Neutral buffered formalin 10% was used to repair rat tissues (Sigma- Aldrich). The tissues were paraffin-fixed, sliced into slices that were 5 m thick, and stained with hematoxylin and eosin. 40X magnification was used to inspect the tissue.
Statistical analysis
The results were represented as mean standard deviation (SD) values and each group was separately examined using one-way ANOVA and a post-hoc Dunnett T-test with GraphPad Prism software 7.0.4. Statistical significance was determined by p-value less than 0.05.
Results and Discussion
Percentage Yield (w/w) of extraction
The percentage yield in (w/w) of both Tinospora cordifolia stem extract and Alkaloid rich fraction was found to be 29.7% and 18%, respectively.

Body weight changes
Body weight changes were observed in all the animals. Body weight growth in L/L rats became slower after 6 weeks when compared to the control group (body weights of L/L G1, L/L G2, L/L G3, L/L G4, L/L G5, and L/L G6 againstcontro L/Dgroup at the end of 6 weeks: expressed in means ± SD 245.5 ±2.5 g, 244.5 ±2.5 g, 244.75 ±2.75 g, 244.25±2.75 g, 244.5 ±3.5 g, and 244.5 ±2.5 g vs. 249.5± 5.5 g, 𝑃>0.05). The difference in body weights between seven groups were statistically significant after 10 weeks (body weights of the L/L G1, L/L G2, L/L G3, L/L G4, L/L G5 and L/L G6 against control L/D group at the end of 10 weeks; (254 ±4 g, 253 ±4 g, 254 ±4 g, 253.25±4.25 g, 254 ±4 g, and 253 ±4 g vs. 265.± 5 g, 𝑃< 0.05) and persisted for the duration of the experiment (Figure 2).
Estrous cycle
It is analogous to the menstrual cycle, which is a part of the human reproductive system (ovarian and uterine cycles). The four stages of the estrous cycle Proestrus phase, Estrus phase, Metestrus phase, and Diestrus phaselast for four to five days [17].
Changes in estrous cycle
Vaginal smears were taken daily used to investigate estrous cyclicityat the start, after four weeks and after 16 weeks of the experimental period. The outcomes are displayed in (Figure 3) as follows:
(1) Four consecutive days of vaginal smears were taken before continuous light reveal a disciplinary transformation with the onset of metestrus, diestrus, proestrus, estrus, and in that order. In (Figure 3), diestrus smears were distinguished clearly leukocytes in big quantities, small-nucleated epithelial cells in huge quantities, vast numbers of cornfield epithelial cells in estrus smears, and leukocytes in large quantities in metestrus smears. We noticed that on all four days, the cyclicity in the vaginal smears stopped at a Proestrus stage.



(2) In Tables 2 and 3, estrous cycle disturbances (ECD) at the end of the 4th and 16th weeks, p-values are P= 0.0013** and P= 0.0059**, while Table 3 at the end of treatment, P= 0.0297* and all the results are expressed as mean ± SEM, P < 0.05, respectively.
(3) After the 16th week of induction period 33 out of 42 rats were shown indiscriminate estrous cyclicity, but at the end of treatment, 32 out of 42 showed disciplinary cyclicity Table 4 and Figure 4.
Changes in ovarian weight
There was no difference in ovarian weight between the right and left ovary between both the L/D and L/L groups after 4 weeks of experimental therapy (Figure 5), (P > 0.05).
Changes in uterine weight
At the end of the treatment uterus weight of all the animals were measured. The L/L G2, L/LG4, L/L G5, and L/l G6 rats uterine weights were similar to L/D group (Figure 6) (P < 0.05), but L/LG4 and L/LG6 showed significant with control group.
Hormonal analysis
The blood serum levels of FSH, LH, insulin, estrogen, and testosterone were estimated following the treatment period. When compared to the disease control group, the level of Luteinizing hormone, insulin, and testosterone were considerably lower, while the levels of FSH and oestrogen were significantly higher.
Testosterone levels
Hyperandrogenism is used to diagnose PCOS in women. It is brought on by faulty ovarian or adrenal function, which leads in an overproduction of androgens. The main effect of hyperandrogenism in PCOS is impaired folliculogenesis [18]. Figure 7 displays the changes in testosterone levels, in which treatment groups of L/L G4 TCSE HD L/L G5 ARF LD and L/L G6 ARF HD showed significant (P < 0.05) lower levels when compared with L/L G1 DC disease control group (3.01 ± 0.083 ng/mL).

All the testosterone levels in the above three groups (1.07 ± 0.069 ng/mL, 1.21 ± 0.063 ng/mL, and 1.01 ± 0.063 ng/ml) were returned to normal L/D group (1.14 ± 0.040 ng/mL).
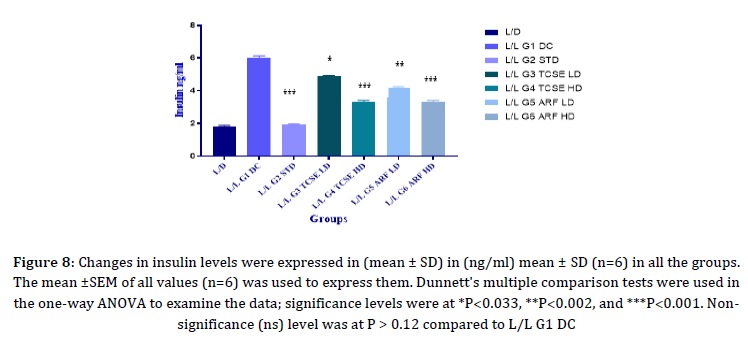
Insulin levels
Since PCOS is tightly correlated with insulin resistance, which could lead to obesity and type 2 diabetes, the insulin levels were measured. In the current study, (Figure 8) represents changes in insulin levels were observed. Significant decreases in insulin levels with p-value < 0.05 were seen in all groups L/D, L/L G2 STD, L/L G3 TCSE LD, L/L G4 TCSE HD, L/L G5 ARF LD, and L/L G6 ARF HD (0.81 ± 0.063 ng/mL, 0.92 ± 0.049 ng/mL, 1.47 ± 0.069 ng/mL, 1.34 ± 0.090 ng/mL, 1.17 ± 0.069 ng/mL, and 0.92 ± 0.088 ng/mL) when compared to L/L G1 DC (4.11 ± 0.098 ng/mL). The body weight changes were measured and noticed in (Figure 2).
Normal follicular development and ovulation are stimulated by follicle stimulating hormone (FSH). FSH controls sex steroid hormone synthesis, oocyte maturation, and folliculogenesis. Women’s with lower FSH levels might indicate that ovaries are not producing enough eggs that lead to ovarian insufficiency [19] eventually that might cause infertility. When a mature egg is released by the ovaries, the FSH levels may be high, but in PCOS, the FSH levels are low, making it difficult to stimulate the growth of the egg and to produce more immature eggs, which develop into cysts in the ovaries and cause PCOS. (Figure 9) illustrates the significant (P <0.05) increases in FSH levels that were found. In contrast to the L/L G1 DC group (0.18 ± 0.06 mIU/mL), high amounts of FSH were found in the L/D (5.6 ± 0.10 mIU/mL), L/L G2 STD (5.3 ± 0.09 mIU/mL), L/L G3 TCSE LD (6.7 ± 0.04 mIU/mL), L/L G4 TCSE HD (6.8 ± 0.06 mIU/mL), L/L G5 ARF LD (6.6 ± 0.06 mIU/mL), and L/L G6 ARF HD (6.9 ± 0.07 mIU/mL).
Luteinizing hormone promotes the releasing of eggs (ovulation). During the ovulatory phase, luteinizing hormone levels will rise. In normally healthy women, the ratio of LH to FSH ranges between 1 and 2. This ratio is reversible in polycystic ovarian disease patients and may even increase to 2 or 3 [20]. In the L/L, G1 DC group clearly shows the elevated levels of LH hormone when compared to L/D group, all the treatment groups’ manifest significant decrease in the levels of LH hormones when compared to disease control group. The substantial (P <0.05) downfall of LH levels observed are shown in (Figure 10).
Estrogen level
Estrogen helps the ovaries create eggs through the process of ovulation, and it also thickens the uterus' endometrium, preparing it for pregnancy. Reduced estrogen levels and elevated gonadotropin levels are associated to the Ultraviolet (UV) radiation exposure [21]. In (Figure 11) L/L G1 DC group markedly shows the reduced levels of estrogen where as in treatment groups L/L G4 TCSE HD, L/L G5 ARF LD, and L/L G6 ARF HD showed elevated levels of estrogen compared to disease control.

Human insulin receptors (INSRs) are crucial a membrane-spanning glycoprotein found on all cells and a member of the tyrosine kinase receptor family [22]. Exon 17–21 encoding the protein tyrosine kinase domain of INSR gene is necessary for insulin action. Any imbalance in the abundance or functionality of INSR might impair insulin's ability to do its function, leading to insulin resistance and type 2 diabetes (T2D) [23]. Insulin resistance affects more than 50% of PCOS patients, which are thought to have a 5- to 8-fold higher risk of developing T2D [24]. PCOS has been linked to the polymorphism rs1799817 (C/T) in exon 17 of the INSR gene (His1085His) [25]. Regarding the PCOS clinical symptoms, INSR has been proposed as a viable candidate gene. The genetic alteration of insulin signalling in the brain has revealed that this system is crucial for controlling ovulation and body weight [26]. Whereas genetic polymorphism or variations in exon 17 of the INSR gene is linked to insulin resistance and hyperandrogenism [27]. Hence, downregulation of INSR gene contributes to insulin resistance [28]. In our findings, observed that in the disease control group, INSR gene expression was down regulated hence M2 group showed insulin resistance, whereas M5 and M7 showed significant up-regulation of the INSR gene represented in (Figures 12 and 13). High doses of T.Cordifolia and Alkaloid rich fraction effectively up-regulated INSR gene so that insulin receptors might increase the sensitivity of insulin signalling.
In Figure 8, the insulin levels where decreased due to continuous light exposure after treatment with high doses of T.Cordifolia and Alkaloid rich fraction in Figure 13, INSR gene was upregulated, it may increase the sensitivity of insulin and reversed the insulin resistance.



Estimation of melatonin by HPLC
The endogenous hormone melatonin, a biogenic amine, is synthesized by the pineal gland in response to darkness and reduces when exposed to the light. Melatonin is also synthesized in female reproductive organs such as follicular cells, oocytes, and cytotrophoblasts. Melatonin is primarily a potent scavenger of free radicals with protective role on female reproductive system. For instance, it aids in shielding the oocytes during ovulation from oxidative stress [29]. Gonadotrophin production is impacted by melatonin insufficiency, which also impacts FSH and LH synthesis, the latter being a most notable change in PCOS patients [30]. The levels of melatonin were considerably lower in individuals (PCOS) with greater LH: FSH ratios than in individuals with lower ratios, showing an inverse relationship between LH: FSH ratio and melatonin production [31]. Light exposure alters the circadian cycle of melatonin and suppresses melatonin synthesis acutely [32].
The HPLC analysis for the melatonin presence in stem extractof Tinospora cordifolia and alkaloid rich fraction of low dose and high doses, L/D, L/L G1 DC, L/L G2 STD, L/L G3 TCSE LD, L/L G4 TCSE HD, L/L G5 ARF LD, and L/L G6 ARF HD was performed. The process was based on acetonitrile extraction, water separation of the chemicals by HPLC, and diode array detector detection. The HPLC chromatograms showed the presence of a peak with a melatonin-like UV-Vis absorption spectrum. The sample name led to the identification of the substance as melatonin. HPLC analyses were carried out on each Sample. The retention time (in min) (RT) of extracts; namely, L/D (2.779), L/L G1 DC (3.008), L/L G2 STD (2.971), L/L G3 TCSE LD (2.793), L/L G4 TCSE HD (2.804), L/L G5 ARF LD (2.638), and L/L G6 ARF HD (2.760), correlated well with that of Melatonin standard only with negligible differences illustrated in (Figure 14). Of the seven samples, L/L G6 ARF HD 2.612±0.0124 µg/g was found to have higher Melatonin content. Followed by L/L G4 TCSE HD 2.55±0.0474 µg/g, L/L G5 ARF LD 1.261±0.1609 µg/g, L/D 2.519±0.016 µg/g, L/L G3 TCSE LD 1.81±0.0432 µg/g, L/L G2 STD 2.471±0.0030 µg/g, and the lowest amount in L/L G1 DC 0.431±0.060 µg/g, (Table 5) is shown. The hormone melatonin, whereby the pineal gland secretes throughout the biological night, serves as the body's internal biological signal for darkness. Both melatonin's circadian rhythm and its synthesis are altered by contioniousexposure to light. The high doses of stem extract ofTinospora cordifolia and alkaloid rich fraction (L/L G4 TCSE HD and L/L G6 ARF HD) groups restored melatonin levels to L/D control group.
Histopathological examination
The histopathological examination of the ovaries (left and right) shown in (Figure 15) indicated that different types of follicles were present and counted, as presented in the (Table 6). They were enumerated according to their follicular type, which included cystic follicles, atretic follicles, and corpus luteum. In comparison to the L/L G1 PCOS group, the number of cyst follicles were considerably reduced in all therapy groups (L/L G2 to L/LG6). There was a significant decrease in the atretic follicle in the (L/L G2 to L/LG6) compared to the L/L G1 PCOS group, but there was no significant difference between the L/D group and the L/L G1 groups.



The corpus luteum was also counted which was significantly higher in (L/L G2 to L/LG6) compared to the L/L G1 PCOS group. The reduction in cysticfollicles clearly represent the role of Tinospora cardifolia against PCos.
Conclusion
A common endocrine issue that affects in women is PCOS, may make it difficult to conceive. With low side effects, herbal therapy has a promising future in the PCOS treatment. In previous studies herbal medication rich in antioxidants, boosts human defence, and immune system. Tinospora cordifolia stem extracts and Alkaloid rich fraction significantly corrected, hormonal imbalance, irregular cyclicity, and ovarian physiology against continuous light induced PCOS. Restoring melatonin levels and upregulating INSR gene expression might reverse insulin resistance, and it also corrects the hormonal imbalance, hence facilitating the restoration of a normal estrous cycle and may alleviate the clinical manifestations of polycystic ovary syndrome.The LH, insulin, and testosterone levels were considerably lower in the treatment groups, whereas FSH and estrogen levels were significantly higher in L/L G4 TCSE HD and L/L G6 ARF HD when compared to all other groups. 32 out of 42 rats exhibited disciplinary estrous cyclicity at the treatment completion. We noticed that the INSR gene had significantly expressed less bright in the M5 and M7 groups than in the disease control (M2 group) representing Tinospora cordifolia may have a capacity to act on INSR gene. Melatonin levels were considerably increased in both L/L G4 TCSE HD and L/L G6 ARF HD. Hence, our findings suggesting that higher doses of stem extracts of Tinospora cordifolia and alkaloid rich fraction will correct sleep disturbances by elevating melatonin levels and insulin resistance by up-regulation of INSR genemay have better ameliorative and therapeutic effect in the treatment of circadian rhythm disorder, in women’s those who working shift at night time, and insulin resistance associated PCOS.
Acknowledgments
The authors would like to thank for the advanced instrumentation facility. “TRI-BIOTECH,” Trichy Research Institute of Biotechnology Pvt. Ltd., for providing HPLC Instrumentation Facility and INSR gene expression. Sincere thanks to Dean Dr. V.Chitra for the extensive support and motivation for this research work.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Orcid
Murali Krishna Moka
https://orcid.org/0000-0002-3654-2798
Sumithra M
https://orcid.org/0000-0003-1665-0190
HOW TO CITE THIS ARTICLE
Murali Krishna Moka, Dr. Sumithra M. Upregulation of INSR Gene Expression and Restoration of Melatonin Levels by Tinospora cordifolia Stem Extract and Alkaloid Rich Fraction against Continuous Light Induced Polycystic Ovary Syndrome in Rodents. J. Med. Chem. Sci., 2023, 6(9) 2198-2217


