Document Type : Mini-Review Article
Authors
- Ali Mohammed Abd AL-Ameer 1
- Lubna Abdulazeem 1
- Mazin H. Kzar 2
- Essam Shawky Khattab 3
- Ahmed Samir Naje 4
1 DNA Research Center, University of Babylon, Babylon, Iraq
2 Department of Physical Education and Sport Sciences, Al-Mustaqbal University College, Babylon, Iraq
3 Chemistry Department, College of Science, Al-Azhar University, Egypt
4 College of Engineering, Al-Qasim Green University, Babylon, Iraq
Abstract
For everyone, at all ages, to live healthy lives and to promote well-being, there should be good health and welfare at every stage of one's life, beginning at birth, whereas health and wellbeing are crucial. Strong health systems are essential for treating and preventing infectious illnesses as well as providing mothers and children with life-saving treatments. Having a well-trained health workforce, a robust infrastructure, a consistent supply of medicines and equipment, and the ability to quickly identify and address health emergencies are all components of a strong health system that provides care to those in need regardless of where they live or their financial situation. Under ideal conditions, cells of the innate immune system detect the danger signals provided by developing tumors. These signals induce hypertrophy, activate innate effector cells with antitumor activity, stimulate antigen-presenting cells on endogenous tumor cells or antigens, and then travel through lymph nodes to inform adaptive (T and B) lymphocytes. Despite this excellent screening process, tumor presence suggests that the advanced tumor somehow avoids detection or overburdens the immune response. Cancer cells have developed a number of strategies that help them evade or resist the host's immune response. Through understanding the mechanism by which these cells evade the immune response, scientists hope to devise techniques to increase tumors immunity and the success of their treatment. In this review, we try to explain the ways and strategies that cancer cells have used to evasion from immune system in an attempt to understand these mechanisms and try to find standard solutions, therapeutic measures, and targets have developed or are investigated to promote tumor rejection.
Graphical Abstract
Keywords
Introduction
What the immune system does?
The immune system protects the body from bacteria, viruses, fungi, and parasites that cause infection and illness. It is a collection of the body's reactions and responses to the damaged or infected cells. As a result, the immunological reaction is sometimes used for its description. For cancer patients, the immune system is critical because:
- The immune system can be weakened by cancer.
- As a result of cancer treatment, the immune system may be impaired.
- The immune system may aid in the battle against cancer.
In general, the immune system in the body is not only striving to battle illness and injury, but also attacks body members that do not work in a correct manner, as in cancer cells with their own tricks on the immune system to evade it, remain alive, and produced proteins stop any part of the immune system is fully capable of eliminating the tumor that has formed [1]. It reads the receptors that are foreign to the body which appear on the cells covers, “Non-self receptors,” and shines on them. Its immune mechanisms, such as T-lymphocytes stick to the tumor cell to secrete weapons and enzymes on it, which destroys tumor cells into small crumbs that the body gets rid of later [2]. When tumor grows significantly, the immune system almost keeps pace with this growth rate by destroying the tumor cells, as illustrated in Figure 1.
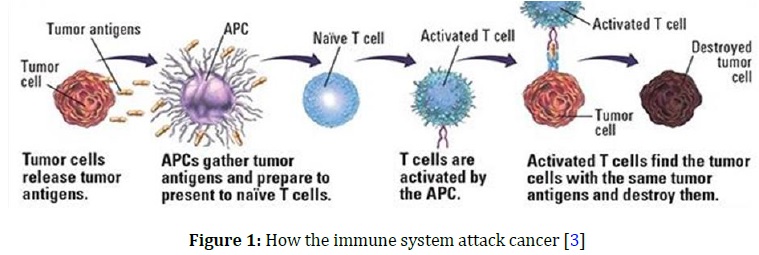
The tumor growth rates equal the ability of immune system to eliminate tumor, so the tumor size remains constant and when tumor continues to grow beyond the ability of immune system to keep pace, in this case, tumor increases in size and turns into a cancerous tumor [4], and during its growth, it develops various means to deceive the immune system, it may display receptors on its cell membranes similar to those found in normal cells “Self receptors”, or hide. The receptors are entirely on its cell membrane. Therefore, the immune system is not even aware of its existence, while some tumors secrete messages to the immune cells to tell them - metaphorically - to stop working, spreading, and fighting the body, which allows cancer to grow and spread. For tumor to live and spread, it needs many malicious measures to adapt itself to the body so that it does not recognize it as a foreign body, and then turns on it to benefit from it and completely paralyzes its movement so that a place can be prepared for it to graze in as it pleases [5]. Thus, tumor cells cooperate, understand and fully integrate, not only in maintaining their malignant tumor entity, but also in developing many ways to help them penetrate into the body through the secretion of enzymes breaking down the spaces between cells to pass through and the secretion of proteins stimulating the formation of blood vessels from which they can move into the bloodstream to the other members [6]. Finally, tumor cells secrete substances that increase their growth and activity, and also the other substances that paralyze the movement and activity of the immune cells so that they can live at the expense of the cells of the body, safe from the immune system [7]. The evasion process of the immune response called tumor escape can result from the emergence of one or more of the following mechanisms.
Mechanism of evading cancer cells from immune system
Downregulation of MHC class I molecules
Adaptive immunity is the foundation of immunological memory. Once our immune system has responded to a certain disease, it can create a quick and precise response if it encounters the same intruder again. MHC class 1 is an essential component of this immune system wing. Most cells have MHC class 1 on their surfaces. They exhibit sections of internal cellular proteins on the cell surface, giving an indication of the cell's health. T lymphocytes kill a cell if their protein flags are aberrant. Although the connection between MHC class 1 and T cells has been thoroughly documented, the role of macrophages has remained a mystery. The histocompatibility complex, it plays a role in presenting what is happening in the manufacture of proteins inside cells and presenting them on the cell surface in the form of peptides. Tumor-inducing viruses have evolved ways to decrease class I MHC expression and assembly with peptides thereby blocking the presentation of the viral antigen to T toxic cells [8]. The viruses that down regulate the presentation of peptides to T toxic cells, include adenovirus that down regulates the transcription of class I MHC molecules, herpes simplex virus inhibits peptide transporter, TAP, associated with antigen processing. These strategies are operative in a normal viral infection and a virally induced tumor. Even when tumors are not virally induced, tumor cells show down regulation of class I MHC molecules or β2-microglobulin or TAP, or some component of proteasomes. All these changes result in a decreased presentation of peptide to T toxic cells and the resulting tumor becomes resistant to T toxic cells [9]. In vitro systems have shown that an increased expression of class I MHC molecules on tumor cells (by IFN-γ) results in an increased susceptibility of these cells to T toxic cells in vitro [10], as depicted in Figure 2.

Blocking of T cytotoxic response by antibodies
Tumor cells and host cells infected with intracellular pathogens are cytotoxic to CTLs. These cells have two functions: they express the CD8 coreceptor and they kill infected cells in an antigen-specific way dependent on MHC class I molecule expression on APCs.
Key points about cytotoxic T cells
▪ CTLs, like TH cells, recognize antigen in the context of MHC class I and are fully activated by accessory costimulatory molecules.
▪ CTLs directly destroy target cells by triggering apoptosis. They release preformed perforins at the target cell surface, causing transmembrane holes to develop in the target cell, allowing a second set of proteins and granzymes to enter the cytosol and trigger an apoptotic cascade.
▪ Surface-bound chemicals can potentially be used by CTLs to send apoptotic signals.
Tc in some cases, antibodies formed against a tumor-bearing host may bind to tumor antigens, effectively blocking epitopes from T cytoxic cells [12]. This blocking effect can be mediated by anti-tumor antibodies alone or by antigen–antibody complex. These complexes may also bind NK cells or macrophages and may inhibit ADCC (Antibody-depending cellular cytotoxity).
Modulation of tumor antigens
Many human malignancies are either weakly or completely non-antigenic. Antigenic modulation may also occur in tumor cells. In the presence of serum antibody, several tumor-specific antigens have been found to vanish from the surface of tumor cells. Such “antigen loss variants” are usually detected in rapidly dividing tumors [13]. Due to high mitotic rate of tumor cells and their genetic instability, these antigen loss variants arise. If these antigens are not essential for the growth or survival of the transformed phenotype, these antigen loss variants have growth advantage in the host, and hence survive and proliferate.
Lack of co-stimulators
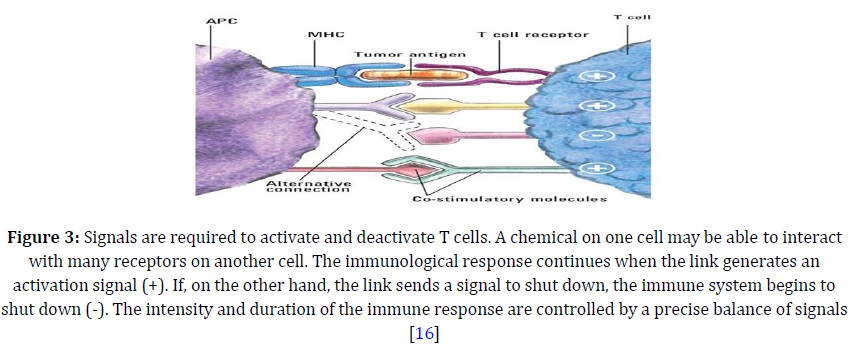
T-cell activation necessitates both an activating signal-triggered binding of the peptide-MHC molecule by TCR and a costimulatory signal-triggered binding of the antigen-presenting cell's B7 with T cells. Both signals are required for T cells to become activated and proliferate [14]. If tumor cells are B7-negative, that is, they lack costimulator molecules, and then the maximal anti-tumor Tcyt-cell differentiation, and hence effector T-cell response does not occur. B7-negative tumor cells when transfected with genes for B7-1 and B7-2 molecules [15], are able to elicit strong cell mediated immune response, as indicated in Figure 3. Once stimulated, these Tcyt cells can act on B7-negative tumor cells, as the effector phase of Tcyt killing does not require co-stimulation.
Suppression of anti-tumor immune response
Some tumor cells express/produce immunosuppressive tumor products such as transforming growth factor β (TGF-β) in a large quantity, to inhibit cell division and the effector function of lymphocytes and macrophages. Other tumor cells express Fas ligand (FasL) that recognizes the Fas molecule on leukocytes [17]. When FasL binds Fas, it results in the apoptotic death of the leukocytes, as illustrated in Figure 4.
Antigen masking
The cell surface tumor antigen may be hidden from the immune system by glycocalyx molecules such as sialic acid. This phenomenon is called antigen masking and occurs because tumor cells exhibit more glycocalyx (surface carbohydrate) molecules than normal cells [19]. Similarly a tumor cell may shield itself from immune system by activating coagulation cascade and coating itself with fibrin [20]. The anti-tumor antibodies or the specific T cyt cells which are formed fail to react with the concealed tumor cells, making the tumor resistant to immune attack.

Preventing inflammatory response
Some tumors prevent the triggering of an inflammatory response by secreting cytokines/growth factors such as IL-10 [21] or the vascular endothelial growth factor (VEGF) [22], that interferes with dendritic-cell activation and differentiation or blocking the production of pro-inflammatory molecules by the tumor cells.
Creating a harsh tumor environment
Tumor through cancer cells can manipulate cellular metabolism by rapidly depleting some amino acids in the tumor environment, and one of these amino acids is tryptophan [23], which effect of this including inhibition of tumor-specific T-cell infiltration, induction of T-cell proliferative arrest, anergy and apoptosis, as well as promotion of T reg cell differentiation and function.
Immune checkpoint signaling
Immunological checkpoints are receptor-based signaling cascades that suppress T cells and induce immune tolerance, allowing malignancies to avoid immune surveillance and escape detection. CTLA-4, PD-1, and PD-L1 are the most well-known of these checkpoints, as displayed in Figure 5. Monoclonal antibodies that block immunological checkpoints have emerged as a game-changing cancer treatment Immune checkpoint therapy has recently been broadened to treat various cancers after proving to be an effective treatment for advanced [24], unresectable melanoma.

Figure 5: When the CTLA - 4 molecule attaches to other molecules instead of them, the T Cell receives a signal to shut down (-) (left). When PD - 1 links to PD - L1, the T cell receives a signal to shut down (-), and it becomes inactive (right) [25]
Conclusion
From the above mentioned points, it is clear that cancer cells have developed extraordinary skill not to fight, but to defend and escape so as not to cost themselves much. Scientists in this field of research are still waiting to reveal other methods that may lead to a more understanding of how cancer cells overcome the immune response, and thus may help design effective immunotherapies against tumors. However, it has become clear that no treatment response is uniform, highlighting the need for individualized tumor analysis and the corresponding individual immunological/immunogenetic background to decipher, on the one hand, the specific pathways used by the tumor to thwart the immune system of hosts and, on the other hand, the latter's potential responsiveness. Immunotherapy should be synthetized with other pillars of cancer treatment, such as surgery, chemotherapy, and radiation to greatly increase the impact of each therapeutic modality.
Acknowledgements
The authors would like to express our gratitude to all members of the research team, particularly the Dean of Al-Mustaqbal University College, represented by Assist. Prof. Dr. Hassan Shakir Majdy, for the financial assistance offered to finish this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
ORCID
Ali Mohammed Abd AL-Ameer
https://orcid.org/0000-0001-9664-6372
Lubna Abdulazeem
https://orcid.org/0000-0003-1572-7395
Mazin H. Kzar
https://orcid.org/0000-0001-8341-6842
Ahmed Samir Naje
https://orcid.org/0000-0001-8997-4087
HOW TO CITE THIS ARTICLE
Ali Mohammed Abd AL-ameer, Lubna Abdulazeem, Mazin H. Kzar, Essam Shawky Khattab, Ahmed Samir Naje. Mechanism of Immune System for Evading and Escaping Cancer Cells: A Brief Review. J. Med. Chem. Sci., 2023, 6(8) 1843-1850


