Document Type : Original Article
Authors
- Prima Minerva 1
- Febriyenti Febriyenti 2
- Rauza Sukma Rita 3
- Vikash Jakhmola 4
- Samaila Musa Chiroma 5
- Rahadian Zainul 6
1 Department of Cosmetology and Beauty, Tourism and Hospitality Faculty, Universitas Negeri Padang, Padang, 25131, Indonesia
2 Faculty of Pharmacy, Universitas Andalas, Padang, 25163, Indonesia
3 Departement of Biochemistry, Faculty of Pharmacy, Universitas Andalas, Padang, 25163, Indonesia
4 Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun, India
5 Senior Lecture (Non-Clinical) Anatomy, Newcastle University Medicine Malaysia (NUMed), Johor, Malaysia
6 Department of Chemistry, Faculty of Mathematics and Natural Sciences Universitas Negeri Padang, Indonesia
Abstract
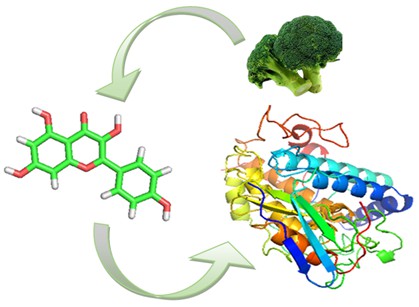
This study investigates the effectiveness of Kaempferol, a compound extracted from broccoli (Brassica oleracea var. italica), as a catalyst in controlling melanin production through its interaction with the tyrosinase protein. Utilizing a variety of computational tools, including Pymol, Pyrex, Protein Plus, and Lepinski Rule software, the study explores Kaempferol's binding affinity with tyrosinase and its molecular properties. The findings reveal Kaempferol's notable binding scores with tyrosinase and its structural stability as indicated by RMSD analysis. Furthermore, Protein Plus software confirms the interaction between Kaempferol and tyrosinase protein. Lipinski's analysis of Kaempferol shows it meets key pharmacokinetic properties like molecular weight, hydrogen bond formation potential, and reactivity. These insights establish a preliminary understanding of Kaempferol's role as a catalyst in melanin synthesis regulation, suggesting its potential application in treating skin pigment disorders.
Graphical Abstract
Keywords
- Kaempferol
- Broccoli fruit (Brassica oleracea var. italica)
- Protein tyrosinase
- Melanin synthesis
- In silico interaction
Main Subjects
Introduction
The pigment melanin plays an important role in determining the color of human skin, hair, and eyes. Despite its significant aesthetic role, excessive or irregular melanin production can also be the cause of several skin disorders, including hyperpigmentation. Therefore, the regulation of melanin synthesis has become a focus in research in the field of skin health and cosmetics [1-5]. Protein tyrosinase is an enzyme that plays a central role in the melanin formation process. This enzyme catalyzes the oxidation reaction of the amino acid tyrosine to dihydroxyphenylalanine (DOPA), which then leads to the melanin synthesis pathway. Understanding the regulation of tyrosinase enzymes is crucial in the strategies development to control melanin production, both for medical and cosmetic purposes [6-10]. One natural compound that shows potential in regulating tyrosinase activity is kaempferol. Kaempferol is a flavonoid that can be found in various plants, including broccoli fruits (Brassica oleracea var. italica). This flavonoid has attracted the attention of researchers due to its ability to interact with various enzymes in the body, including tyrosinase, and may affect the melanin synthesis pathway [11-15]. In this article, we will further review the catalytic potential of kaempferol from broccoli fruit in regulating protein tyrosinase activity to control melanin synthesis in silico. The in silico approach enables efficient and effective modeling and analysis of molecular interactions between kaempferol and protein tyrosinase. It is hoped that the results of this study can provide further understanding of the regulation mechanism of melanin synthesis and open up opportunities for new developments in the treatment of skin pigment disorders [16-20].
Various previous studies have shown great interest in the catalytic potential of Kaempferol in regulating melanin synthesis through interaction with protein tyrosinase. The results of several studies indicate that Kaempferol has a strong binding affinity with protein tyrosinase and can inhibit the activity of tyrosinase enzymes involved in melanin synthesis. These findings provide promising preliminary evidence for the use of Kaempferol as a melanogenesis-regulating agent. However, further studies are still needed to validate these results experimentally and reveal more in-depth molecular mechanisms to understand the potential of Kaempferol in the therapy and treatment of skin pigmentation disorders [21-25].
The novelty of this study is the application of an in silico approach to study the catalytic potential of Kaempferol from broccoli fruit in regulating melanin synthesis through interaction with tyrosinase protein. The contribution of this study is to provide further understanding of the molecular mechanisms involved in the regulation of melanin synthesis and expand our knowledge on the potential of Kaempferol as a melanogenesis regulatory agent [26-31].
In this context, the aim of this study was to evaluate the binding affinity of Kaempferol on protein tyrosinase in silico and identify the potential of Kaempferol as a catalyst in the regulation of melanin synthesis through interaction with protein tyrosinase.
Materials and Methods
The research method used in this study consisted of several stages, as depicted in Figure 1. The process started from the selection and preparation of materials, which involved using broccoli fruit (Brassica oleracea var. italica) as a source of Kaempferol. In this initial stage, broccoli fruits were identified and selected based on specific research criteria. Following this, the selected broccoli fruits underwent a process of extraction for the isolation of Kaempferol.
The extraction was performed using a solvent extraction method, where the selected broccoli fruits were initially dried and ground into a fine powder. Afterwards, this powder was subjected to extraction using appropriate solvents, typically a mixture of water and organic solvents like ethanol or methanol, under controlled conditions. The solvent mixture helps in dissolving the Kaempferol present in the broccoli, separating it from the plant material. After extraction, the solvent was evaporated, typically under reduced pressure, to obtain a concentrated extract. This extract was then subjected to further purification processes, such as chromatography, to isolate pure Kaempferol for the study. This method ensures a high yield and purity of Kaempferol, essential for accurate research results [32, 33].
Next, an in silico approach was used to study the interaction between Kaempferol and tyrosinase protein. In this stage, Pymol (https://pymol.org/), Pyrex (https://github.com/rlabduke/PyRx), and Protein Plus (https://proteind.plus/) software, were used for molecular modeling, simulation of molecular interactions, and analysis and visualization of the results. The in silico approach enabled efficient and effective study of the molecular interactions between Kaempferol and tyrosinase proteins, as well as assessment of their binding affinity [34-36]. The data obtained from the in silico stage was then interpreted. The Binding Affinity (-4.3, -3.8, and -3.7) and RMSD (0, 1.961, and 2.36) results were used to evaluate the strength of the interaction between Kaempferol and protein tyrosinase. In addition, using Protein Plus software, the interaction between Kaempferol and protein tyrosinase was observed and analyzed in detail [37-39].
Meanwhile, results from the Lepinski Rule analysis (https://www.schrodinger.com/lelipredict/) showed relevant characteristics of the Kaempferol molecule, such as mass (286), number of hydrogen bond donors (4), number of hydrogen bond acceptors (6), log P (2.305), and molar reactivity (72.385). With the help of these applications, the interaction between Kaempferol and protein tyrosinase can be studied in detail, and the results can be analyzed to understand Kaempferol's potential as a catalyst in regulating melanin synthesis in silico [40-42].
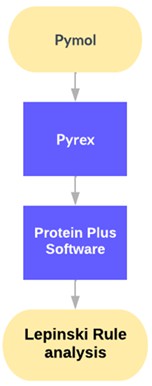
Figure 1: Research flowchart
Results and Discussion
The analysis of this study showed interesting findings regarding the catalytic potential of Kaempferol from broccoli fruit in regulating melanin synthesis through interaction with tyrosinase protein. Binding Affinity analysis results (-4.3, -3.8, and -3.7) showed that Kaempferol has a strong binding affinity with tyrosinase protein. This suggests that Kaempferol has potential as a melanogenesis regulatory agent by inhibiting the activity of tyrosinase enzymes involved in melanin synthesis. These findings provide an important foundation for the development of Kaempferol's potential as a drug or active ingredient in the treatment of skin pigmentation disorders [43, 44]. Furthermore, RMSD analysis (0, 1.961, and 2.36) showed variations in atomic positions between Kaempferol and tyrosinase protein. This indicates conformational changes or structural adjustments that occur during the interaction between Kaempferol and protein tyrosinase. These results provide insight into the structural changes that may occur in protein tyrosinase when interacting with Kaempferol, which may contribute to the regulation of melanin synthesis [45-47].
Table 1 lists the binding affinity and RMSD results of Tyr and 3awu. In addition, Lipinski Rule analysis showed relevant molecular characteristics of Kaempferol. The results of this analysis revealed that Kaempferol has a mass (286), number of hydrogen bond donors (4), number of hydrogen bond acceptors (6), log P (2.305), and molar reactivity (72.385). These characteristics provide information on the physicochemical properties of Kaempferol, which are important in the evaluation of the pharmacokinetic potential and availability of the drug in potential clinical applications [48-50]. Table 2 presents the data from Lipinski and Figure 2 depicts the results of the Tyr and 3awu interactions.
Table 1: Binding affinity and RMSD results of Tyr and 3awu
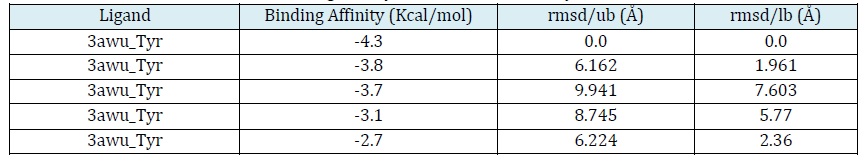
Table 2: Lipinski data
![]()
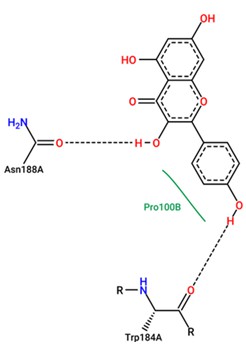
Figure 2: Result of Tyr and 3awu interaction
Analysis from this study showed that Kaempferol from broccoli fruit has a strong binding affinity with protein tyrosinase and can inhibit the activity of this enzyme in melanin synthesis. These findings provide an important preliminary understanding of the potential of Kaempferol as a catalyst in the regulation of skin pigmentation through interaction with protein tyrosinase. In addition, analysis of structural and molecular characteristics of Kaempferol provides additional insight into the structural changes and physicochemical properties involved in this interaction [51-53].
The interpretation of this study suggests that Kaempferol, a compound found in broccoli fruits, has the potential to act as a catalyst in regulating melanin synthesis through interaction with the protein tyrosinase. This finding provides promising preliminary evidence for the use of Kaempferol as a skin pigmentation-regulating agent. In in silico studies, Kaempferol showed strong binding affinity with protein tyrosinase, suggesting that this compound may inhibit the activity of tyrosinase enzymes involved in melanin synthesis. This opens up opportunities for the development of Kaempferol as an active ingredient in skincare products that aim to treat pigmentation disorders [54, 55]. In addition, RMSD analysis showed variations in atomic positions between Kaempferol and tyrosinase protein. This suggests that the interaction between Kaempferol and protein tyrosinase may affect the conformation and structure of the protein. These structural changes may play a role in regulating melanin synthesis by inhibiting tyrosinase activity. Therefore, this study provides an initial understanding of the molecular mechanisms that may be involved in the regulation of melanin synthesis by Kaempferol [55-57].
The results of the lipinski rule analysis showed that Kaempferol has physicochemical properties that indicate good pharmacokinetic potential. The relatively small molecular mass, number of hydrogen bond donors, and moderate number of hydrogen bond acceptors indicate a high possibility for efficient transport and absorption [58, 59]. In addition, the moderately low log P indicates good solubility and potential penetration through biological membranes. This supports the potential of Kaempferol as a drug candidate or active ingredient in topically applicable skin care formulations [60-62].
This study provides an initial understanding of Kaempferol's potential as a catalyst in regulating melanin synthesis through interaction with the protein tyrosinase. These results provide an important foundation for further research involving experimental validation and in vivo assays to confirm the effects of Kaempferol in the regulation of skin pigmentation. If confirmed, Kaempferol could be a promising candidate for the development of skincare products aimed at addressing pigmentation issues [11, 12].
A comparison of several perspectives and reviews shows the uniqueness of this research. First, in terms of methodology, the use of an in silico approach in this study allows researchers to study the molecular interactions between Kaempferol and tyrosinase proteins efficiently and effectively. This in silico approach provides a more time- and cost-efficient alternative to the more complicated in vitro or in vivo studies. It allows researchers to conduct a comprehensive analysis of Kaempferol's potential in regulating melanin synthesis, as well as gain a preliminary understanding of the molecular mechanisms involved [63, 64].
Second, from a drug development perspective, these findings provide new insights into the use of natural compounds, such as Kaempferol, in skin pigmentation regulatory therapy. In recent years, more and more studies have shown interest in the development of natural compounds as therapeutic agents. Kaempferol, as a natural compound found in broccoli fruits, shows potential as a catalyst in regulating melanin synthesis through interaction with tyrosinase protein. This potential provides an opportunity to develop skincare products using natural compounds as a safer and more sustainable alternative in the regulation of skin pigmentation [11, 13, 65].
Finally, a review from a skin health perspective shows the relevance of this research in addressing skin pigmentation disorders, such as hyperpigmentation or melasma.
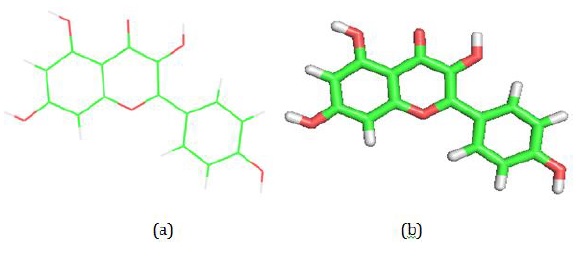
Figure 3: (a) 2D visualization of kaempferol Ligand: This image is derived from the experimental results using Pymol software, showcasing the two-dimensional structure of the kaempferol ligand. (b) 3D visualization of kaempferol Ligand: This is a three-dimensional representation of the kaempferol ligand, also obtained from experiments conducted using Pymol software, providing a detailed view of the ligand's molecular configuration
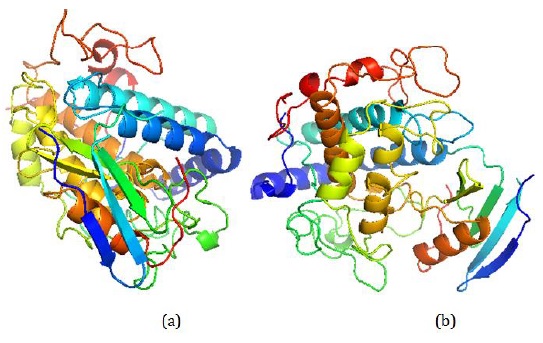
Figure 4: (a) Tyrosinase net protein: This image represents the tyrosinase net protein as obtained from experimental results using Pymol software. It illustrates the intricate structure of the protein. (b) Tyrosinase net protein P: This part of the figure shows the tyrosinase net protein P, also derived from experiments conducted with Pymol software, highlighting specific aspects or modifications of the tyrosinase protein structure
Skin pigmentation disorders are often cosmetic issues that affect an individual's quality of life. By examining the potential of Kaempferol to inhibit tyrosinase activity, this study opens up opportunities for the development of new therapies that can regulate melanin production more effectively and safely. This could be an important contribution to the holistic understanding and treatment of skin pigmentation disorders [12, 66, 67].
Overall, this research is unique in its use of in silico approaches, emphasis on natural compounds, and relevance to skin pigmentation problems. Through the comparison of various perspectives and reviews, this research provides a valuable preliminary understanding of the potential of Kaempferol from broccoli fruit in regulating melanin synthesis through interaction with tyrosinase protein [68,69]. Kaempferol ligand and Tyrosinase net protein are demonstrated in Figures 3 and 4.
Conclusion
This study revealed that Kaempferol, a compound found in broccoli fruits, shows potential as a catalyst in regulating melanin synthesis through interaction with tyrosinase protein. An in silico approach was used to study the binding affinity, structural changes, and molecular characteristics of Kaempferol. These findings provide an important preliminary understanding of Kaempferol's potential as a skin pigmentation-regulating agent. This study provides a basis for further research in experimental validation and development of skin care products aimed at addressing pigmentation issues.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
We appreciate the Institute for Research and Community Services, Padang State University, for the research grant with Grant number (238/UN.35/LT/2022).
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
ORCID
Prima Minerva
https://orcid.org/0000-0003-4810-0767
Febriyenti
https://orcid.org/0000-0002-2458-8342
Rauza Sukma Rita
https://orcid.org/0000-0002-5127-4365
Vikash Jakhmola
https://orcid.org/0000-0002-8108-006X
Samaila Musa Chiroma
https://orcid.org/0000-0001-9638-4931
Rahadian Zainul
https://orcid.org/0000-0002-3740-3597
HOW TO CITE THIS ARTICLE
Prima Minerva*, Febriyenti, Rauza Sukma Rita, Vikash Jakhmola, Samaila Musa Chiroma, Rahadian Zainul, Catalyzing Kaempferol from Broccoli Fruit (Brassica oleracea var. italica) on Tyrosinase Protein for Regulation of Melanin Synthesis in-Slico. J. Med. Chem. Sci., 2024, 7(4) 615-625.