Document Type : Original Article
Authors
1 Medical Department, Chair of Clinical and Laboratory Immunology, Allergology and Medical Genetics, Bogomolets National Medical University, Kyiv, Ukraine
2 AirDOC Allergy and Immunology Clinic, Kyiv, Ukraine
Abstract
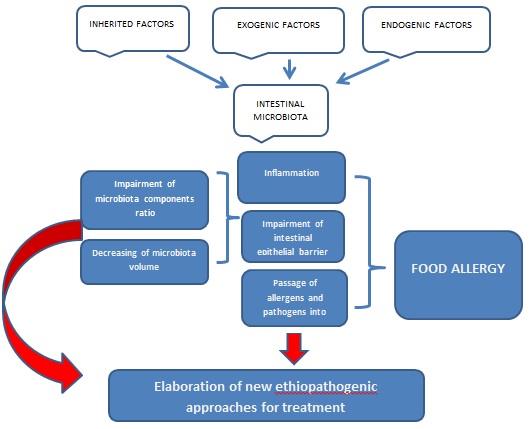
Nowadays the problem of food allergy is of great importance. It impairs the quality of life of people all over the world. People suffer from food allergies starting from the earliest period of life. Usually, people get antihistamine remedies with a variety of side effects. Food allergy may be caused by various allergens and its development depends on different factors. The aetio-pathogenic treatment used to be elaborated. Gut microbiota plays a crucial role in maintaining of homeostasis of any organism with a gastro-intestinal tract. Human beings are not exceptions. The human gastrointestinal tract is enormously colonized by various microorganisms, which localize predominantly in the colon in a symbiotic relationship with the host. Impairment of microbiota function causes different diseases of the host including food allergy. The work aims to analyse the literature of the last decade (2013-2023) to reveal the connection between the condition of microbiota and its role in the development of food allergy. Scientific articles from MEDLINE-PubMed, Embase, and Cochrane databases were analysed in the work. A set of "MeSH terms" was created to remove a large number of irrelevant papers during the manual search. It was settled that the content of microbiota and its functional activity is of great importance for food allergy development both in infants and adults. The main problem lies in the synthesis of the short-chain fatty acids and their interrelation. The characteristics of the colon epithelial barrier, its adhesive qualities, and permeability, which depend on the functional activity of microbiota, also influence on development of food allergy. Intestinal microbiota is essential for maintaining of intestinal epithelial barrier, which plays one of the pivotal roles in intestinal health and provides homeostasis keeping. Microbiota destruction by antibiotics or other exogenic/endogenic factors leads to impairment of the intestinal epithelial barrier, which in turn serves as a trigger of inflammation and allows pathogens and allergens passage from the intestinal cavity into the bloodstream.
Graphical Abstract
Keywords
Introduction
Food allergy is a common immunologic reaction to different kinds of food. It begins predominantly at an early age and is usually connected with immunoglobulin E (IgE). IgE-mediated food allergy is becoming increasingly common, but the etiopathogenesis remains unknown to date. There are two main hypotheses for the formation of food allergy: genetic and environmental influences, which have been identified as risk mediators for food allergy. The human gut microbiota produces many different substances that can enter the bloodstream and affect distant organs and systems. The human gut microbiome is one of the most actively studied microbial communities. This is due to the incredible complexity of its composition and the abundance of its interactions with the human body. A great number of different hypotheses about the involvement of the intestinal microbiota in the pathogenesis of various diseases are increasingly being formulated. The formation of the human intestinal microbiome is a multi-step process. About 90 % of the total intestinal microbiota is bacteria of Firmicutes and Bacteroidetes types, mainly represented by hard-to-cultivate obligate anaerobes. In European populations, the most frequent and numerous representatives of Firmicutes are Faecalibacterium prausnitzii and bacteria of the genera Blautia, Dorea, Roseburia, and Coprococcus, while the main representatives of intestinal Bacteroidetes include bacteria of the genera Bacteroides, Parabacteroides, Prevotella, Odoribacter, Barnesiella, and Alistipes. Bacteria of the types Actinobacteria make up a small percentage of the intestinal microbiota of adults, while Fusobacteria, Verrucombicrobia, and methanogenic archaea of the type Euryarchaeota make up an even smaller proportion of Proteobacteria [1]. Microbial colonization etiopathogenetically may account for increased susceptibility to foods and play a role in the mechanism of food allergy development.
That is, despite splendid theories of food allergy development and, elaboration of various remedies aimed at diminishing its symptoms, nowadays the problem of food allergy takes a prime place among modern diseases and needs to be solved. Intestinal microbiota influences greatly the homeostasis of human beings and its role in the etiopathogenesis of different diseases is indisputable. Therefore, it is necessary to verify the role of intestinal microbiota in food allergy development.
Literature review
Microbiota components
The term microbiota defines all microorganisms, not only bacteria, but also fungi, protozoa, and viruses, present in our body. In particular, the human gastrointestinal tract is colonized by approximately 1013-1014 microorganisms, of which 15,000 different bacterial strains localize predominantly in the colon in a symbiotic relationship with the host [2]. In addition to the dominant family of microorganisms, bacteria, the gut contains core-containing eukaryotes and prokaryotes, which lack a DNA-containing nucleus, as well as viruses, and protozoa. Collectively, the human gut microbiota contains about 3.3 million genes, which is about 150 times more than the number of genes in the human genome. In young children, the colon microbiota is dominated by bifidobacteria, accounting for up to 95% of the total microbial population [3]. In children aged 8-16 months, the predominant strains of bifidobacteria are Bifidobacterium longum (76%), B. bifidum (52%), B. catenulatum (21%), B. breve (21%), B. adolescents (10%), and B. dentium (7%) [3]. Age-specific combinations of dominant strains of bifidobacteria have been established. B. longum subsp. infantis, B. animalis subsp. lactis, B. breve, and B. bifidum characteristics for children in the first year of life have anti-inflammatory effects and promote the formation of a Th1 (T-helper1) -immune response. The predominant strains of bifidobacteria in adults - B. adolescentis, B. longum subsp. longum, B. pseudocatenulatum - promotes a Th2 (T-helper2) -immune response and predominates in the gut microbiota in obesity. Throughout the growth and development of the child, and its maturation, the ratio of the main classes of microorganisms changes in favor of Bacteroidetes and Firmicutes. It was found that 4 bacterial phyla - Firmicutes (F), Bacteroidetes (B), Proteobacteria (P), and Actinobacteria (A) - prevail in the intestinal ecosystem in terms of content and number of constituent species: they account for more than 95% of the identified taxa. The phylum Fusobacteria, Verrucomicrobia, and Cyanobacteria accounted for 1 to 5%. Data on the proportion of representatives of other communities capable of being both symbionts and parasites of humans (archaea, fungi, viruses, helminths, and protozoa), amounting to about 0.2%, are not final. So far, they are referred to as the "dark matter" of microbiomes and even attributed to them the problems of lack of response to probiotic interventions (due to possible chronic inflammation during host cell invasion) and reduced biodiversity (due to phage exposure) [4]. Generalization of data on the biology of phylum representatives showed that Firmicutes (F) are characterized by active metabolism of carbohydrates, including non-digestible polysaccharides, as well as protein, Bacteroidetes (B) – degradation of protein and amino acids, polysaccharides, bile acids, choline, Actinobacteria (A) – hydrolysis of complex carbohydrates, and Proteobacteria (P) are heterogeneous in nutritional type, often utilizing and producing intermediate metabolites [5, 6]. Thus, the B: F index, which shows the ratio of total Bacteroidetes to Firmicutes abundance, is a fairly constant value at different ages, varying in parallel with α-diversity. In turn, this ratio is modified only with physiologically driven age-related changes in feeding patterns [7]. In normal children in the first year of life, the B: F index is at the level of 0.4 with the lowest diversity due to the predominance of actinobacteria (up to 90%) against the background of monotonous, at least before the introduction of complementary feeding, milk feeding. In adults with full nutrition reaches 11 (with a steady increase in α-diversity), and after 65 years sinusoidally decreases to 0.6 under the influence of factors associated with its deterioration in old age due to dementia, hypochlorhydria with alkalinization of chyme and shutdown of microbial enzymes with an optimum pH≤6.0 drop in the activity of somatic enzymes and absorption [8]. Normally, the microbiota is characterized by a predominance of obligate-anaerobic representatives of Firmicutes and Bacteroidetes types, ensuring gut health and general condition, while the disturbance of homeostasis, known as dysbiosis, associated with proliferation of certain bacterial populations such as Enterobacteriaceae or lack of momentous commensal bacteria contributes to a more favourable environment for the pathogens growth that predispose the body to pathological conditions [9, 10]. According to what was recently reported in a study conducted by the Human Microbiome Project (Human Microbiome Project) and the European Meta HIT Consortium, the bacterial flora of the human gut, despite being composed of a very large number of different species, can be divided into the three most represented genera: Bacteroides and Prevotella belonging to the type Bacteroidetes, and Ruminococcus belonging to the type Firmicutes [11]. During the time from birth to adulthood, the microbiota undergoes many changes. In fact, the neonatal microbiota is prematurely formed by E. coli, while a newborn passes through the maternal birth canal; later on one can define Bacteroidetes, Bifidobacteria, and Clostridia which inhabit a newborn during the first days, weeks after birth and it turns to be stable similar to adults only at the age of 2-3 years [12]. The intestinal microbiota fulfils various significant functions to maintain host homeostasis. By synthesis of short-chain fatty acids, it forms and maintains the epithelial barrier predominantly in the colon. This synthesis occurs due to the fermentation of undigested substances, as well as through immunostimulation and immune tolerance, drug, and toxin metabolism [13].
Functions of microbiota
The formation of intestinal microbiocenosis occurs mainly during the first year of a child's life, depends on the nature of nutrition and numerous environmental factors. During the same period of life, immunologic tolerance to microorganisms inhabiting the intestine develops. The microbiota that make up the microbiocenosis of various loci of the GI tract are not perceived by the immune system of the macroorganism as foreign, but acquire antigenic properties of the organ [14]. Members of the intestinal mucosal microbiota constantly interact with the intestinal immune system through epithelial-recognizing -Toll-like receptors. As a result, a unified microbial-tissue complex is formed, which includes bacteria, mucosal epithelial cells and stromal cells, exopolysaccharides, glycocalyx, and mucus. Due to the constant exchange of genetic material, molecules, plasmids, intestinal microbiota, acquiring antigens and receptors inherent in the macroorganism, ceases to be foreign, is not rejected and is not subjected to phagocytosis. Microorganisms of the normal microbiota contribute to the maturation of the intestinal immune system, model the cytokine profile, and activate the synthesis of secretory immunoglobulin (Ig) A. Normal microflora of the child's intestine in the postnatal period stimulates the direction of the immune response of T helper cells toward the Th1 variant, with subsequent balancing of Th1, Th2, and Th3/Th1 immune responses. The protective function of intestinal microbiota also consists in the formation of colonization resistance, strengthening of the intestinal barrier, synthesis of mucin, deconjugation of bile acids, and improvement of epithelial regeneration. Representatives of the intestinal microbiota, attaching to the receptors of intestinal epithelial cells, prevent colonization of exogenous, pathogenic microorganisms, and compete with opportunistic bacteria. The intestinal microbiota, synthesizing disaccharidases, is able to break down dietary fiber, plant polysaccharides, and synthes short-chain fatty acids, thus performing not only digestive but also the function of a "metabolic organ". In early childhood, when bifidobacteria predominate in the microbial landscape, short-chain acetic acid (acetate) is produced in greater quantities, which enhances the barrier function of the intestinal epithelium and has an anti-inflammatory effect. As the child grows and develops, with a change in the nature of nutrition, the introduction of complementary foods, the proportion of obligate anaerobes in the intestinal microbiota increases. The intestinal microbiota acquires an "adult type", in which the production of other short-chain acids, such as butyrate and propionate with protective, anti-inflammatory functions, affect immunity, modify the cytokines production by lymphocytes, begins to prevail. The GI microbiota has a detoxifying function towards numerous exogenous and endogenous substrates and metabolites. In particular, the obligate microbiota inhibits histidine decarboxylation, reducing histamine synthesis and the risk of toxicoallergic reactions. In the process of vital activity, bacteria are able to neutralize or utilize nitrates, xenobiotics, and mutagenic steroids from the external environment. The mechanisms which maintain equability between the intestinal microbiota and the host are the followed ones: production of gastric acid, mucus, bile salts; mucosal pH, mucosal Ig, local, and systemic immunity, interrelations between various microbial species and intestinal peristalsis. Altered microbiota-host relationships can potentially lead to gastrointestinal or extraintestinal diseases, which are determined as "gut microbiota-related diseases" such as: different allergic diseases, intestinal and bowel inflammation, obesity, metabolic syndrome, diabetes mellitus both of 1 and 2 types, and diseases of cardiovascular and bony systems, such as osteoporosis [2].
Food allergy and gastro-intestinal tract
Food allergy affects about 10% of the population; it prevails mostly in childhood and is typical for the countries with low income. It can be defined as an unexpected altered immune reaction on the base of immunologic impairment, when a specific response of immune system is caused by consummation of food antigens usually tolerated by the majority of people [15, 16]. The most popular foods, which trigger allergic reaction are peanuts, cow's milk, chicken eggs, nuts, fish, shellfish, wheat, seeds, and soy [17]. Oral tolerance is able to prevent the development of food allergy; it is mediated by dendritic cells which influence on the differentiation of naive T cells into Foxp3+ T cells, able to produce IL-10. As a result one can find an inhibition of sensitization to food allergens. Intestinal epithelial cells synthesize pro-inflammatory cytokines, which influence on antigen-presenting cells, increase Th2 cells number, they synthesize IL-4 and due to this stimulate the allergic response, isotypic switching of B cells toward IgE production. Eosinophils and mast cells expansion also take place. The outbreake of food allergy is stipulated by the impaired tolerance to ingestion. Specific IgE produced during the first stage of sensitization after prime antigen contact. It attaches to specific receptors on basophils and mast cells, and then when the second contact with the food allergen takes place, basophils and mast cells due to the binding of food antigen to IgE, synthesize different substances including histamine, TNF-α, platelet activating factor, leukotrienes, IL-4, IL-5, IL-9, IL-13, IL-31, and IL-33 that results in a variety of symptoms including anaphylaxis [18]. Nowadays, nearly one-third of the population believes that they suffer from food allergy; however, the actual data based on physician diagnosis is 5% of adults and 8% of children [17]. Usually the foods cause IgE mediated food allergy are milk, egg, peanuts, tree nuts, wheat, seafood, etc. [19]. IgE-mediated food allergy and food induced-anaphylaxis are the most well-known and well-characterised type food allergy prevailed in children of 0-3 years old [20] which demonstrates an increasing tendency in the Western countries [21]. Such allergy is characterised by immediate and reproducible paroxysms of allergic reactions, caused by food-specific IgE. This diagnosis of food allergy may be confirmrd by in vivo or in vitro tests [20]. On basophils, mast cells, dendritic cells, and Langerhans cells high-affinity receptors for the Fc region of IgE are expressed, their ligation with specific IgE results in histamine and other mediators release by activated mast-cells, which causes typical hyperactivity reactions such as low blood pressure, vasodilation, consequent bronchospasm, hives, etc. [22]. The immune response of hyperactivity reaction is rapid; the symptoms are usually revealed in between 5 to 60 min after food ingestion, nevertheless they are rarely followed by fatal anaphylactic outcome. A rare form of IgE-mediated food allergy is a pollen-associated type of food allergy which develops because of the cross-reaction between allergen molecules in exact pollens and some fruits and vegetables [23]. Usually it does not result in anaphylaxis. This reaction may be avoided by boiling of the fruit or vegetables or by peeling their skin. This kind of food allergy is usually misdiagnosed. Non-IgE food allergy has been increasing worldwide. It is presented by variety of gastrointestinal disorders, commonly of slow onset, such as colic, gastroesophageal reflux, diarrhoea, etc. making it difficult to verify its diagnosis [24]. Mixed IgE-cell-mediated food allergy, is characterised by involving of both IgE and immune cells in the reaction, it manifisttes by gastrointestinal tract (eosinophilic esophagitis and gastroenteritis) or skin (atopic dermatitis) symptoms [25]. The aim of the work is to provide deep analysis of literature concerning the microbiota role in development of food allergy.
Material and Methods
For this comprehensive search for relevant articles, the MEDLINE-PubMed, Embase, and Cochrane databases were searched for relevant studies starting in 2013. A set of "MeSH terms" was created to remove a large number of irrelevant papers during the manual search: "microbiota/food allergy"[Mesh], "microbiota/short-chain fatty acids"[Mesh], or " short-chain fatty acids / food allergy "[Mesh]), or "microbiome / food allergy "[Mesh], or "microbiota/ IgE food allergy "[Mesh], or "microbiota/Non-IgE food allergy "[Mesh], or "microbiota/epithelial barrier”. The search terms and strategies were similar in the study of another database (EBSCOhost).
Results and Discussion
Nowadays there is worldwide approved data concerning gut microbiota contribution to the author-pathogenesis of food allergy [26-31]. Bacteroidetes and Firmicutes form merely 90% of the microbiota and influence on development of food allergy. Clostridia species are capable of increasing Treg cell production, which inhibits allergic reactions and increases oral tolerance. Bacteroides fragilis can produce polysaccharide A, which increases Treg cell cytotoxic activity and IL-10 production by these Foxp3+ T cells. However, there is data which underlines an insignificant connection between bacteria of the Clostridia class, Firmicutes type, in healthy children and ones with food allergies [10]. In the investigation conducted by Fazlollahi M. et al. 141 children with egg allergy were studied. It was settled that the prevalence of Lachnospiraceae, Streptococcaceae, and Leuconostocaceae in the gut microbiota of patients. Therefore, the obtained results allow investigators to conclude that the presence of Lachnospiraceae and Ruminococcaceae families in children's gut-microbiota as a predisposition for the development of egg-associated food allergy and make them a target for further interventions: preventive or therapeutic [32]. Intestinal microorganisms such as Clostridium septum, Eubacterium rectal, and Faecalibacterium prausnitzii synthesize short-chain fatty acids (fermentation produces butyrate, propionate, acetate, and valerate). Colon is the place of short-chain fatty acids with higher concentration. These short-chain fatty acids provide direct immunomodulatory effects and are important for immunologic tolerance development, except for short-chain fatty acids synthesis gut-microbiota also secret spermidine, spermine, putrescine, and cadaverine – polyamines which maintain the epithelial barrier. Nowadays the hygiene hypothesis exists. It means that the lack of antigens, which meet newborns and infants in early childhood, antibiotic administration, and diet alternate gut-microbiota and suppress the development of the immune system and leads to the onset of atopic diseases, including food allergy [10]. After complementary feeding, the content of the microbiota changes due to the diet used with obvious differences in composition between dietary fibre, characterized by Alistipes, Bilophila, and Bacteroides, and higher levels of Roseburia, Ruminococcus and Eubacterium, and a fat-rich diet. Thus, Lactobacillus, Clostridium, Ruminococcus, Ruminococcus, Peptostreptococcus, and Bacteroides are bacteria which regulate immune response and T-lymphocyte proliferation with subsequent induction of IL-10R1 receptor expression and inhibition of pro-inflammatory cytokines through tryptophan catabolization [33]. Among the most important mechanisms of immune response formation to food allergens the state of the intestinal wall, epithelial barrier and cavity microflora take the first place. More importantly, the intestinal mucosa has lymphoid tissue, which is the largest accumulation of immune cells in the human body. By interacting with this lymphoid tissue, the intestinal microflora takes an active part in the formation of the local and systemic immune response to food allergens. In a healthy person, the immune reaction caused by food allergens is provided by the presence of specific immunologic reactivity to antigens, immunologic reactivity to food antigens, and autoantigens with preservation of immune reactions against pathogens. Qualitative and quantitative changes in the qualitative and quantitative composition of wall and cavity intestinal microflora can provoke an inadequate immune response to food allergens. Conditionally pathogenic microflora may contribute to sensitization to food allergens by increasing the permeability of the intestinal wall and affecting the differentiation of T-helper cells. Therefore, it is understandable that a high-fat but low-fibre (dietary fibre) diet, such as the Western diet, may be responsible for the development of food allergies in Western countries [34]. The "nutrition-gut microbiome-physiology axis", is defined. It means a substantial connection between diet, gut microbiota and allergic reactions. Greater Foxp3 expression is associated with food diversity. Hence, one can suggest that a diverse diet may provide a protective effect against food allergy development. In contrast, in children with less diverse diets, reduced expression of Foxp3 was revealed [2]. In the experiment antibiotic treatment leads to allergisation of mice in comparison with control ones. This fact underlines the role of microbiota in the prevention of allergy development. It was shown that eosinophil-deficient germ-free mice were inclined to intestinal fibrosis and were less prone to allergic sensitization compared to control ones. These results also stress that commensal bacteria regulate the function of intestinal eosinophils, which alter tissue repair and cause allergic sensitization to antigens including food ones. So, interactions between the commensal microbiota and intestinal eosinophils are crucial for the maintenance of homeostasis, and innate and adaptive immunity in health and disease [35]. More frequently, Clostridiales and Lactobacillales provide a valuable effect on food tolerance. However, Bacteroidales and Enterobacteriales provide an ambivalent effect. The gut microbiota of children with atopic dermatitis and food allergy is represented by Escherichia coli and Bifidobacterium pseudocatenulatum and less by Bifidobacterium adolescentis, Akkermansia muciniphila, Bifidobacterium breve, and Faecalibacterium prausnitzii in comparison with children with atopic dermatitis without food allergy. Thus, the authors found a connection between early colonization with potentially more pathogenic microorganisms, such as Clostridium difficile or Stafilococcus aureus, and food allergy development. In contrast, colonization with more valuable microorganisms, such as bifidobacteria, is associated with food tolerance [2]. However, there is data which reveals the desensitizing effects of Lactobacillus rhamnosus GG in allergic reactions to cow's milk and peanuts [10, 36, 37].
The World Allergy Organization (WHO) for probiotic supplementation concluded that there is a small risk reduction of food allergy signs that may be associated with probiotics [38]. It was settled that, in addition to the known local effects (trophic, anti-inflammatory and anticarcinogenic effect on colonocytes, acidification of intestinal contents), 95% of short-chain fatty acids are absorbed into the blood and influence physiological processes occurring in the liver, white and brown adipose tissue, bone marrow, lungs, pancreas, and intestine. Through binding to histone deacetylase receptors (inhibition), receptors conjugated to the G-protein-mediated family of intracellular signalling cascades (activation) and peroxisome proliferator-activated receptor γ receptors of these organs and systems, they regulate fat metabolism, carbohydrate metabolism, energy metabolism, cellular immunity responses, etc. [39, 40]. All short-chain fatty acids participate in the regulation of immune responses and energy consumption in the body. The ability of acetate to reduce lipolysis and accumulate fat in white adipose tissue, butyrate to reduce adipocyte size and increase lipolysis in brown adipose tissue, propionate to regulate gluconeogenesis in the liver and along with butyrate to activate intestinal gluconeogenesis was described in detail. Acetate helps reduce food and energy intake by interfering with appetite and satiety regulation in the hypothalamus by inducing the synthesis of incretin hormones by enteroendocrine cells in the gut [41, 42]. Furthermore, in response to gut-microbiota interactions, it stimulates the synthesis of anti-inflammatory cytokines and sIgA via the G protein-coupled receptor GPR43, which induces the generation of regulatory T lymphocytes (Treg) in the periphery and their differentiation. Being incorporated into glucose, fatty acids and molecules of cholesterol, acetate and propionate penetrate with them into organs and tissues, including the central nervous system [40]. The described discoveries allowed us to understand the true biological meaning of fermentation in the large intestine of the largest by volume component of the diet – resistant starch and non-starch polysaccharides of plant origin. It consists in the permanent formation, with strict regularity in all individuals, irrespective of geography, of a certain set of short-chain fatty acids, in which acetate, propionate, butyrate prevail, and their entry into the bloodstream as essential signalling molecules – regulators of energy metabolism, immunity, and metabolism [5, 39]. The constancy of this constellation has been confirmed in prospective studies showing that the proportion of genes for plant polysaccharide metabolism, synthesis of some amino acids and vitamins, and methanogenesis is dramatically increased in the intestinal flora of inhabitants of different regions of the world [58]. It has been proved that disturbances in short-chain fatty acid production as a result of dysbiosis can lead to negative consequences for the host. Thus, high acetate production by microbiota in a rodent model promoted metabolic syndrome [5, 39, 40]. The revealed regularities finally connected the nutriome and microbiome into an inseparable whole, proving the urgency of regular consumption of cereals, vegetables and fruits with a daily diet necessary in terms of volume and caloric content to ensure proper endogenous synthesis of SGC. According to the data of modern identification, the producers of the main SGCs in the intestine are mainly representatives of obligate anaerobic saccharolytics, including butyrate – Faecalibacterium, Ruminococcaceae and Lachnospiraceae sp., propionate – Bacteroides, Propionibacterium, Roseburia, Selenomonas sp., acetate – Bifidobacterium, Clostridium, Ruminococcus, Lactobacillus sp., and acetate – Bifidobacterium, Clostridium, Ruminococcus, and Lactobacillus sp. However, mutual transformations and the ability of proteolytic microbes to synthesize isomeric forms of branched-chain short-chain fatty acids from proteins of animal origin and glycoproteins, the biological significance of which is ambiguous, have also been described. Therefore, the evaluation of metabolites, without analysis of microbial sources of their synthesis and causes of disorders that determine the development of a particular pathology, may be unpromising for their correction. One of the causes of disorders may be the imbalance of sugarolytics with methanogens – hydrogen utilizers, and this determines the importance of studying, along with bacteria producing short-chain fatty acids, as well as the populations responsible for the community balance. In the same context, the relevance of taxonomic characterization of the microbiome is important for assessing the state of innate immunity (which is provided and maintained by the physical interaction of microbial bodies and structures with epitheliocytes and lymphoid apparatus of the intestine, production of somatic, capsular, flagellar, membrane antigens expressing various factors of local and systemic immunity), and the inflammatory status based on the endotoxin dynamics. Short-chain fatty acids exert biological activity through multiple mechanisms. Initially, they can be used by human cells as energy sources in the process of oxidative phosphorylation. In particular, butyric acid can provide 60-70% of the energy requirements of colonocytes [43]. Secondly, short-chain fatty acids inhibit histone deacetylases, which has an anti-inflammatory effect: as a result of their action, the level of transcription carried out by NF-κB family factors is reduced, the level of tumour necrosis factor-α produced is decreased, and FoxP3+ Treg cells maturation is induced [44]. Thirdly, these substances are specific ligands of several G-protein-coupled receptors - GPR41, GPR43 and GPR109A [43, 44]. The maturation and function of microglia, dendritic cells, and Treg cells are regulated through these receptors [44]. The action of short-chain fatty acids is not limited to the influence on the functioning of immune system. For example, they induce the proliferation of intestinal Vocaloid cells and increase mucin production [44]. As substrates for gluconeogenesis and lipogenesis, they participate in the regulation of carbohydrate and lipid metabolism in liver cells [43].
The multiple functions of the human microbiota, as well as their impact on human health, have predetermined a large number of studies on the composition of the microbiota in various diseases. First, it concerns pathologies of the digestive system, in particular, functional gastrointestinal disorders (regurgitation, colic, and stool disorders) - the most common problem in children, especially among infants in the first year of life. Thus, various forms of functional gastrointestinal disorders are observed in more than half of children under 6 months of age. The role of the microbiota in the physiology and pathology of the human digestive system is extremely large. Some metabolites produced by probiotics, such as butyric acid, are important energy substances for epitheliocytes. In particular, short-chain acids produced by microorganisms have a stimulatory effect on sensitive receptors of the enteric nervous system, which regulates the state of the microbial-tissue complex of the intestinal mucosa. Deficiency of short-chain acids or disturbance of their composition changes the functional state of the intestinal wall and enteric nervous system. Excess microbiota growth promotes the development of chronic mucosal inflammation; disruption of mucosal homeostasis is considered an important component of the pathogenesis of functional gastrointestinal disorders. One of the reasons for the high prevalence of functional gastrointestinal disorders in young children may be the delayed formation of microbiota in modern environmental conditions. Thus, according to some data, disorders of intestinal microbiota composition are found in 3/4 of children over 2 years of age living in a large industrial city. Deviations are manifested by the deficiency of bifidobacteria and lactic acid streptococci, and the presence of 1-2 species of opportunistic flora. Excessive growth of such species of opportunistic flora as staphylococci, enterococci, Klebsiella, Proteus, and fungi of the genus Candida is observed in half of healthy children under 6 months of age. Increased Escherichia coli in combination with decreased levels of lactobacilli can provoke excessive gas formation in the intestine and discoordination of intestinal motility. The role of gut biocenosis disorders in the development of food allergy in infants is undeniable. Newborn children from the risk group for allergy development with subsequently realized allergic disease have a reduced content of bifidobacteria and predominance of clostridia in contrast to children who did not develop allergy. There are also differences in the strains of bifidobacteria: children with atopy are characterized by intestinal colonization with B. adolescentis, while B. breve, B. infantis, and B. longum are more common in healthy children. Altered microbiota, an impaired intestinal epithelial barrier seems to be a background of food allergy. The epithelial barrier is a functional unit that equalises the antigenic charge of the intestinal lumen and immunological and non-immunological complexes of the intestinal mucosa. In general, it provides nutrient absorption and protects the body from the permeability of harmful macromolecules. It was settled that the functioning of tight junctions is regulated by cytokines produced in the gut and can be impaired by different factors such as consumption of alcohol, nutritional imbalances and bacterial toxins action [45]. Impairment of the epithelial barrier and gut microbiota causes the development of gut inflammation, leading to food allergy and estrogen loss. It was found that hormone depletion may increase gut inflammation due to a greater antigenic load crossing the epithelial barrier [45]. Estrogens appear to play a dual role in promoting allergic disease and mast cell degranulation due to allergen exposure. Oral contraceptive use is implicated in the aetiology of urticaria and chronic angioedema [45]. Furthermore, the intestinal microbiota is capable of influencing circulating estrogen levels through the secretion of β-glucuronidase, an enzyme that activates them. The integrity of the epithelial barrier, usually caused by the presence of four types of microorganisms: Bacteriodetes, Firmicutes, Actinobacteria, and Proteobacteria, is altered in dysbacteriosis, in which a decrease in intercellular junctions increases intestinal permeability, leading to bacterial translocation that induces a systemic inflammatory state at the base of various pathologic processes (i.e. intestinal bacterial translocation is a migration of viable bacteria from the intestinal lumen to the mesenteric lymph nodes and other extraintestinal organs and sites). In addition, it is important to emphasize that dysbacteriosis leads to decreased deconjugation of estrogen with decreased circulating estrogen, resulting in CD4+ T-cell activation. An inflammatory process underlies food allergy. Some proinflammatory and anti-inflammatory mediators are essential in the development of food allergy when allergens can heterogeneously stimulate Th1-, Th2-, and Th17-cytokines. There is a hypothesis that both TNF-α and IL-6 are essential in food allergy development [46]. While Nadelkopoulou et al. [47] tried to use IL-10 in the food allergy treatment. The role of IL-33 in food allergy development is discussed [48]; its activity contributes to various allergic reactions through its influence on immune cells: mast cells, eosinophils, Th2 cells, Treg cells, natural killer cells, basophils, dendritic cells, and activated macrophages [47, 48]. A high-fat diet in Western countries is one of the factors that may contribute to the increased prevalence of allergies. Diet-induced obesity has been demonstrated to be a factor in increased susceptibility to food allergy and, in particular, it has been found that the microbiota associated with a high-fat diet can increase the susceptibility to food allergy, suggesting a link between diet, microbiota and food allergy [49]. It has been reported that heat-killed lactic acid bacteria increased the percentage of peripheral CD4+CD25+Foxp3+Treg cells and alleviated symptoms during pollen season when administered to patients with mild pollinosis of Japanese cedar (a form of hay fever caused by the pollen of the Cryptomeria japonica tree - ed.). Although not through the microbiota, IBCs are thought to act directly on the immune system. In food allergyrticular, increased levels of Treg cells along with SCFAs are considered as a promising target for improving both allergy and bone metabolic balance [50]. Roduit et al. analysed SCFA levels in faecal samples from 301 children aged 1 year, reporting that children with the highest levels of butyrate and propionate were less likely to have asthma at ages 3 and 6 years and showed significantly lower allergic sensitization with reduced risk of food allergy and diagnosis of allergic rhinitis. Likewise, it investigated the role of butyrate bacterial production in the gut in the development of allergic reactions during early childhood. It was settled that the basis of allergic sensitization had been represented by the absence of genes encoding key enzymes for butyrate production as well as for carbohydrate breakdown [2]. Vitamin D plays a significant role in the regulation of tight gut contacts, which has led to the hypothesis that its deficiency may compromise barrier integrity or cause changes in the composition of the gut microbiota, increasing the risk of food allergy. Sardecka-Milewska et al. found that children with cow's milk allergy have lower serum vitamin D concentrations than healthy children [51]. The role of vitamin D in the development of food allergy is further supported by Koplin et al. who revealed a remiss association between low serum vitamin D levels and food allergy just in cases with polymorphisms followed by lower levels of vitamin D-binding protein. The influence of vitamin D on food allergy development is due to the ability of vitamin D to induce IL-10 expression by Treg cells, resulting in the development and maintenance of oral tolerance [52]. In general, it is highly important to keep in mind the relationship between microbiota and microRNAs. The significance of the microRNA's role in the development of various pathological conditions, including allergies, is becoming increasingly clear [53-56]. The ability to fully understand the relationship between microRNAs and the microbiota may allow for novel disease markers and pave the way for new targeted and personalized therapeutic strategies [57]. The importance of an unaltered microbiota is emphasized by the fact that the increasing trend of antibiotic use leads to impaired intestinal absorption with a deficiency of minerals important for bone health. On the other hand, antibiotic use jeopardizes the development of oral tolerance mechanisms, which leads to increased development of food allergy. Accordingly, the use of prebiotics and probiotics has been justified for the valuable modulation of the gut microbiota in food allergy. For instance, it was revealed that L. rhamnosus can decrease the expression of TNFα, IL-17, and RANKL in cells isolated from the murine small intestine, providing desensitizing effects in cow's milk and peanut allergy [26]. According to the analysis of current literature, it is settled that the gut microbiota and certain types of bacteria can influence susceptibility to disease, including allergies. In particular, the addition of valuable bacteria and dietary adjustments appear to improve outcomes and prevent the occurrence of food allergies.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the article and agreed to be responsible for all the aspects of this work.
ORCID
Liliia Romaniuk
https://orcid.org/0000-0003-1727-1256
Anastasiia Levchenko
https://orcid.org/0000-0002-3945-6502
HOW TO CITE THIS ARTICLE
Liliia Romaniuk*, Anastasiia Levchenko, Study of the Influence of Intestinal Microbiota on the Immune Response in Allergic Diseases Manifested by Food Allergy and Urticaria. J. Med. Chem. Sci., 2024, 7(3) 565-578.