Document Type : Review Article
Authors
Ophthalmology Department, Dr Soetomo General Hospital, Faculty of Medicine, Universitas Airlangga, Surabaya, 60132, Indonesia
Abstract
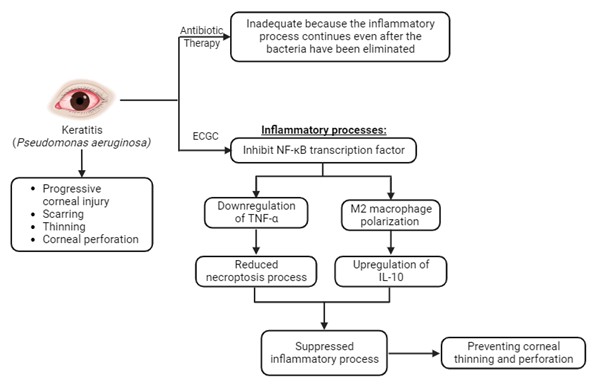
Pseudomonas aeruginosa is the most prevalent cause of keratitis, which can cause progressive corneal injury, scarring, thinning and corneal perforation. Antibiotic treatment alone is inadequate in some cases because the inflammatory process continues even after the bacteria have been eliminated. Anti-inflammatory adjuvant therapy is needed to prevent further complications. Epigallocatechin gallate (EGCG) is the main polyphenol in Camellia sinensis. EGCG has anti-inflammatory and antioxidant properties that are widely recognized. It is known that EGCG acts by inhibiting NF-κB, so it is speculated that the necroptosis process will be reduced due to the TNF-α downregulation and that the wound healing process will begin promptly due to the polarization of M2 macrophages, as indicated by the upregulation of IL-10, therefore, preventing corneal thinning and perforation.
Graphical Abstract
Keywords
Introduction
Corneal inflammation known as keratitis entails the swelling of the cornea, involving the influx of inflammatory cells, corneal edema, and ciliary congestion. This condition arises from both infectious and non-infectious origins [1, 2]. Ninety percent of keratitis cases are caused by bacterial keratitis, with Pseudomonas aeruginosa being the most prevalent pathogen in 23-50% of cases [3]. Pseudomonas keratitis is characterized by necrotizing lesions and a significant accumulation of polymorphonuclear cells, which can cause progressive corneal damage and scar tissue leading to corneal perforation [4, 5].
Pseudomonas aeruginosa is the most prevalent pathogen responsible for bacterial keratitis in the United States (21%), and in Indonesia (24.7%) [6, 7]. P. aeruginosa is responsible for 25% of keratitis cases at Cipto Mangunkusumo Hospital Jakarta over a four-year period and is the second most prevalent cause of bacterial keratitis at RSUD Dr. Soetomo Surabaya by 26% in 2017-2018 [8, 9]. In 12% of cases in India, keratitis caused by drug-resistant P. aeruginosa also causes corneal perforation [10].
In cases of pseudomonas keratitis, it is crucial to administer treatment as soon as possible to prevent permanent blindness. In certain cases, antibiotic treatment alone is ineffective because the inflammatory process persists even after the bacteria have been eliminated. The inflammatory process causes corneal melting, corneal scarring, and corneal perforation, so anti-inflammatory adjuvants are required to treat bacterial keratitis. There are still controversies regarding the use of corticosteroids as an anti-inflammatory adjuvant in bacterial keratitis, and there are no clinical investigations of anti-collagenase in humans [11].
Several polyphenol compounds have been studied as anti-inflammatory, anti-bacterial, and antioxidant agents in various organ diseases, including the eyes. The main polyphenol in tea (Camellia sinensis) leaves is epigallocatechin gallate (EGCG). EGCG has been shown to have anti-inflammatory and antioxidant effects on various types of cells in the body [12]. The EGCG effect on the ocular region, particularly in keratitis, has not been studied extensively. Based on the preceding explanation, the objective of this study is to establish a foundation for the EGCG utilization as an adjunctive anti-inflammatory agent capable of inhibiting inflammatory processes during the acute phase of pseudomonas keratitis. This intervention aims to prevent the progression of corneal damage by elucidating the underlying mechanisms involved. In addition, this study will review and analyze prior research conducted in this area.
Pathogenesis of pseudomonas aeruginosa keratitis
The leading causes of keratitis caused by P. aeruginosa are barrier damage or corneal defects caused by contact lenses cleaner contaminated, eye trauma, and history of eye surgery. Given 1010 bacterial cells of P. aeruginosa or S. aureus, two important pathogens in the cornea, only a few bacteria adhere and none penetrate the stroma in a healthy and intact cornea [3]. In the corneas of experimental animals inoculated with P. aeruginosa, attachment to the damaged epithelium is followed by invasion of the corneal stroma and proliferation, which is frequently exacerbated by host immune responses [3, 13]. Invasion of P. aeruginosa into the stroma is facilitated by microbial proteases with keratolytic action, resulting in liquefactive necrosis of epithelial, stromal, and endothelial cells; exotoxins A and S, which inhibit host protein synthesis; and leukocidin and haemolysin, which create a favorable environment for bacterial infiltration. Bacterial replication and host immune response in the stroma contribute to corneal opacification and loss of corneal transparency [14].
The molecules derived from P. aeruginosa, namely LPS and flagellin, are recognized in the corneal stroma as pathogen-associated molecular patterns (PAMPs). These molecules serve as triggers for pattern-recognition receptors (PRRs), specifically TLR4 and TLR5, situated on macrophages and the other immune cells. This interaction sets off a cascade of inflammatory processes by engaging MyD88 molecules, as illustrated in Figure 1 [13, 14]. Activation of TLR4 and TLR5 within corneal macrophages rapidly triggers the generation of pro-inflammatory cytokines and stimulates chemotaxis-a process involving the influx of inflammatory cells into the corneal stroma and impeding bacterial proliferation and survival. The classic NF-κB pathway is activated by the activation of PRRs, with a specific emphasis on TLRs present on innate immune cells like monocytes, macrophages, neutrophils, and dendritic cells. These receptors discern PAMPs from microbes or DAMPs generated by damaged cells [15-17].
The signaling cascades initiated by TLRs hold a pivotal role in regulating the polarization of macrophages. Notably, the ligand LPS interacting with TLR4 prompts the differentiation of macrophages into the M1 phenotype. This phenomenon of macrophage differentiation is distinctly driven by LPS, which triggers macrophage signaling through the involvement of two separate TLR adapters: MyD88 and TRIF. From a genetic perspective, compelling evidence underscores the indispensability of both the TLR-MyD88-dependent and TLR-TRIF-dependent pathways in dictating the polarization of M1 macrophages, ultimately influencing the expression of pro-inflammatory cytokines [15, 16]. The initiation of the inflammatory response involves the recruitment of several adapter proteins. In the context of P. aeruginosa keratitis, both the MyD88 pathway and the non-MyD88 pathway mediated by TRIF come into play. The MyD88/TRIF pathway triggers the synthesis of CXCL1/KC chemokine, which, in turn, draws neutrophils from the limbal vessels to the cornea. Meanwhile, MyD88 is an essential adapter molecule required for TLR and IL-1R signaling because in animal studies, MyD88 deficiency is associated with decreased expression of cytokines in pseudomonas keratitis, thereby interfering with the innate immune response to infection [16-18].
The CD14 receptor recognizes the intricate structure formed when LPS-binding protein binds to the LPS present in the P. aeruginosa cell wall. This binding prompts CD14 to transfer the LPS to the MD-2 molecule, which is associated with TLR4. Subsequently, MD-2 undergoes structural alterations, triggering the activation of TLR4. Dimerization of TLR4 follows, leading to the recruitment of TIR-domain-containing adaptor proteins (TRIF and TIRAP), initiating the transmission of signals. Activation of TIRAP necessitates the participation of the MyD88 protein, which concurrently enlists IRAK4 and IRAK1 from the IL-1 receptor-associated kinases. Notably, IRAK1 is phosphorylated by IRAK4, setting in motion the activation of TNFR-associated factor 6 (TRAF6) E3 ubiquitin protein ligase.
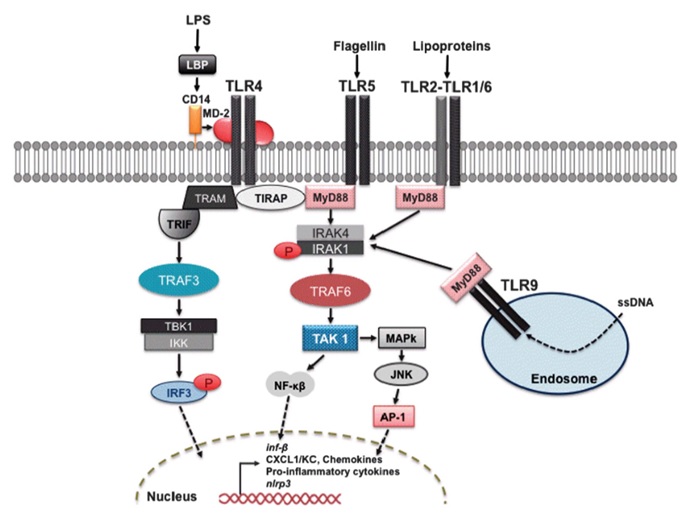
Figure 1: Pseudomonas aeruginosa molecular pattern recognition by the immune system
This, in turn, activates the TGF-β-associated kinase (TAK)1, culminating in the transcription of pro-inflammatory cytokines through the NF-κB and mitogen-activated protein kinase/JNK pathways [13]. NF-κB activation via the MyD88 signaling pathway triggers the synthesis and release of pro-inflammatory cytokines, which encompass TNF-α, IL-1, IL-6, IFN-I, chemokines like IL-8, CXCL1, and CXCL2, in addition to various other inflammatory mediators across various types of innate immune cells [15-19]. Chemokines, arising from NF-κB activation, undertake the crucial role of attracting macrophages and neutrophils to the infection site, where they perform phagocytosis and neutralize P. aeruginosa. Nevertheless, the degranulation of neutrophils, coupled with the discharge of proteolytic enzymes and reactive oxygen species, can lead to substantial tissue damage as part of their strategy to curtail bacterial propagation. Within the cornea, the degranulation of neutrophils triggers the apoptosis of corneal keratocytes and endothelial cells that regulate corneal transparency. This sequence of events eventually culminates in corneal opacification and disturbances in visual perception. Findings from prior studies involving P. aeruginosa infection in healthy mice unveiled an early onset of cytokine production, the mobilization of immune cells into the corneal stroma, and effective control of bacterial replication. These mice exhibited corneal opacities accompanied by concurrent infiltration of neutrophils and macrophages into the corneal stroma, yet corneal perforations were absent. In stark contrast, hindering the TLR or IL-1R1 response at any stage resulted in delayed influx of inflammatory cells, the emergence of corneal opacities, unbridled bacterial proliferation, and eventual corneal perforation [16].
Tumor necrosis factor alpha in pseudomonas keratitis
Originally characterized as a factor capable of inducing tumor necrosis, Tumor Necrosis Factor Alpha (TNF-α) has evolved to be acknowledged as a pivotal modulator of the inflammatory reaction. TNF-α is an inflammatory cytokine produced by neutrophils, activated lymphocytes, macrophages/monocytes, and natural killer cells during acute inflammation; it is responsible for multiple signaling events in cells that result in necrosis or apoptosis. TNF-α was nevertheless detected in normal corneas [20-22].
In the context of pseudomonas keratitis, activation of NF-κB precipitates the synthesis and secretion of pro-inflammatory cytokines, with a notable emphasis on TNF-α. The TNF-α levels exhibit a temporal pattern, surging 6-12 hours post P. aeruginosa infection, reaching its zenith within 1 to 3 days, and subsequently subsiding towards the normal baseline around 5-7 days [21]. In the study by Zuhria et al. (2021) regarding the mechanism of death of corneal epithelial cells due to P. aeruginosa infection, there was an increase in TNF-α as a biomarker that plays a role in the necroptosis-mediated death of corneal epithelial cells [23]. TNF-α propels autocrine TNFR activation, thereby instigating further intracellular communication that facilitates the process of necroptosis-induced cell death. Necroptosis, a regulated form of necrotic cell demise, is governed by kinases RIPK1, RIPK3, and MLKL, as depicted in Figure 2 [24-27].
RIPK1 is responsible for activating RIPK3 within two distinct complexes: the ripoptosome and the necrosome. The active RIPK3, in turn, triggers the activation of MLKL, leading to the onset of necroptosis and the consequent release of DAMPs, which in turn instigate inflammation and activate TLRs. Active TLRs reciprocally activate RIPK3 through TRIF or foster the creation of necrosomes via the release of interferons (IFNs). Additionally, TLR stimulation initiates NF-κB activation by engaging the adapter molecule MyD88. Notably, the active RIPK1-RIPK3 complex also holds the capacity to activate NF-κB, thereby inducing the secretion of TNF and contributing to the formation of both ripoptosomes and necrosomes [24].
Signaling via MyD88 can induce autocrine TNF-α secretion and necroptosis via signaling via TNFR. In studies of mouse fibroblasts, it was determined that RIPK1 plays no role in necroptosis, whereas necroptosis is entirely dependent on RIPK3 and MLKL. Moreover, the activation of necroptosis can further intensify the inflammatory reaction by promoting the synthesis of inflammatory cytokines and releasing DAMPs from necrotic cell lysis-induced membrane damage [15, 24].
Interleukin-10 in pseudomonas keratitis
While anti-inflammatory cytokines like IL-10 are expressed at notably low levels in normal corneas, the infiltration of macrophages into inflamed corneas leads to an elevation in their expression. Both dendritic cells and macrophages demonstrate the capability to produce IL-10 in vitro subsequent to the activation of PRRs. IL-10 can be expressed by dendritic cells, macrophages, and PMNs in vivo. Following stimulation of TLR4 and TLR9, macrophages and myeloid dendritic cells produced a substantial amount of IL-10. Numerous studies underscore that IL-10, recognized as an anti-inflammatory cytokine, emerges as a pivotal regulatory molecule in animal models of P. aeruginosa keratitis, contributing to a favorable prognosis [28, 29].
Upon binding to the IL-10 receptor located on monocytes or macrophages, interleukin-10 initiates the JAK1-TYK2-STAT3 pathway. This cascade results in the transcription of STAT3-mediated genes, which play a role in curbing the inflammatory response by stifling the expression of pro-inflammatory cytokines. Within this framework, the SHIP1 presence is of utmost importance for the function of IL-10. Particularly noteworthy is the formation of the SHIP1-STAT3 complex, which is induced specifically by the anti-inflammatory impact of IL-10. This formation sets apart IL-10 signaling from that triggered by other cytokines that also activate STAT3 [29, 30].
Research involving rats afflicted with P. aeruginosa keratitis has unveiled the role of IL-33 in orchestrating a Th2-type immune response. This response facilitates the dampening of inflammation by steering macrophages towards producing anti-inflammatory mediators, specifically IL-10. This revelation highlights the crucial significance of these cytokines in mitigating disease progression and contributing to the cornea recuperation. Another study with experimental animals B6 and BALB/c rats with P. aeruginosa keratitis evaluated on the third and fifth days after infection, demonstrated the role of macrophages in regulating the resistance of corneal infection caused by P. aeruginosa. This regulation encompasses the modulation of the quantity of polymorphonuclear neutrophils (PMNs), the bacteria-killing capability, and the equilibrium between pro- and anti-inflammatory cytokine levels. Importantly, macrophages are also responsible for controlling the induction of IL-10, a cytokine deemed essential for the healing process of the cornea [28].
Pseudomonas keratitis treatments
Due to their rapid growth, keratolytic enzymes, and stimulation of a detrimental host immune response, pathogenic bacteria can cause irreversible corneal scarring over several hours. Therefore, therapy should be initiated prior to a definitive diagnosis to reduce the load of bacteria and minimize future visual impairment. Initial treatment consisted of broad-spectrum and empiric topical antibiotics. Antibiotic eye drops should be administered every 30 to 60 minutes initially, and then the frequency can be gradually decreased based on clinical response. Antibiotics administered every 5 minutes for 30 minutes as a loading dose can accelerate the achievement of therapeutic concentrations in the corneal stroma in severe cases. After 48 to 72 hours, if keratitis is effectively treated, the majority of infectious keratitis become culture negative [4].
Predominantly, topical antibiotics remain the cornerstone of effective bacterial keratitis management. A recent Cochrane review assessing various combinations of topical antibiotics administered for a minimum of seven days demonstrated that commonly prescribed options exhibit comparable efficacy, irrespective of whether corneal re-epithelialization concludes on time. However, in select cases, the prognosis remains unfavorable due to the inflammatory response culminating in corneal melting, scarring, and perforation [11].
Adjunctive therapy, which includes topical corticosteroids, is employed to curtail the immune response contributing to several complications of keratitis. Despite being a subject of debate, adjuvant steroids are still employed in bacterial keratitis management. These corticosteroids can bolster clinical outcomes by mitigating inflammation, thereby limiting scarring, neovascularization, and stromal degradation, which justifies their administration. Nonetheless, conflicting views persist, with some asserting that corticosteroids might delay corneal epithelium healing and potentially exacerbate the infection. Analyzing four randomized controlled trials encompassing the use of adjuvant steroids for corneal ulcers, a recent review concluded that overall, steroids do not yield significant improvements [11].
Yet another adjuvant therapy in keratitis cases is anti-collagenase treatment. During the acute infection phase, various cells including fibroblasts, keratocytes, and inflammatory cells produce enzymes like collagenase and matrix metalloproteinase (MMP) responsible for protein degradation and keratolysis [31]. This treatment approach seeks to stabilize the condition of corneal melting, thereby reducing the likelihood of severe complications such as corneal perforation and the need for therapeutic penetrating keratoplasty. In a study involving rabbits with pseudomonas ulcers, systemic administration of doxycycline decreased corneal perforation incidents by approximately 50%. However, the absence of human randomized controlled trials hinders the use of doxycycline by corneal specialists as an adjuvant therapy for corneal ulcers, despite its widespread application [11].
Camellia sinensis and epigallocatechin gallate (EGCG)
Tea (Camellia sinensis) is a well-known beverage which is rich in flavonoids, which constitute approximately 30 percent of the dried weight of tea leaves and have several potential health benefits [32, 33]. EGCG (C22H18O11) is the most abundant polyphenol in tea, accounting for approximately one-third of total catechins; from other sources, it accounts for more than 65 percent of catechins and is regarded as a healthy component of green tea. Its therapeutic potential extends to diverse inflammatory conditions including arthritis, atherosclerosis, autoimmune uveitis, endotoxin-induced uveitis, oxidative-induced retinal degeneration, dry eye syndrome, glaucoma, and ocular surface inflammation [34, 35].
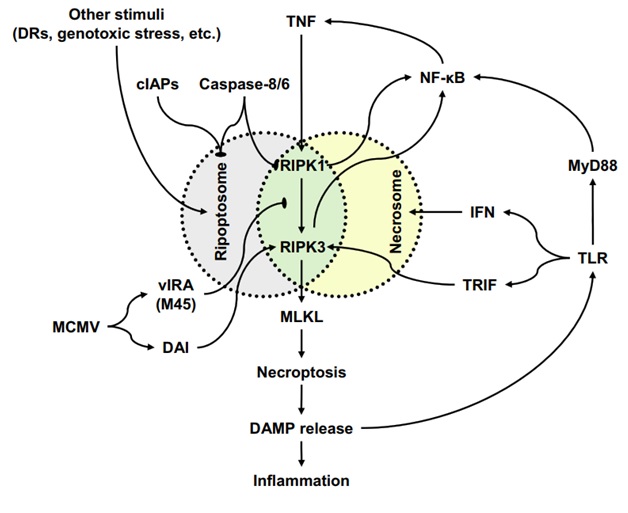
Figure 2: Necroptosis-associated signaling pathways and their interaction
The mechanisms of action employed by EGCG are extensive, mediated through intracellular signaling pathways, nuclear transcription factors, and interactions with cell surface receptors [36]. EGCG's biological potency contributes to the suppression of inflammation, proliferation, and pro-inflammatory cytokines by impeding the NF-κB activation, a group of transcription factors pivotal in immune and inflammatory responses. Inflammatory mediators including pro-inflammatory cytokines like IL-1, TNF-α, Toll-like receptors (TLRs), and reactive oxygen species trigger NF-κB activation [37]. These conditions can decrease levels of the chemoattractant IL-8, which recruits neutrophils and macrophages. EGCG has also been shown to reduce TNF-α levels via NF-κB, thereby inhibiting necroptosis-induced inflammation [38, 39]. Furthermore, EGCG is capable of curbing M1 macrophage polarization while promoting the favorable M2 macrophage polarization, thereby increasing IL-10 expression which promotes tissue repair, remodeling, and wound healing, as well as promoting angiogenesis, fibrosis, and suppressing inflammation [40].
EGCG and ocular inflammations
Numerous investigations, encompassing both in vitro and in vivo methodologies, have been conducted to explore the potential anti-inflammatory properties of EGCG in the context of ocular disease. EGCG has been found to exhibit inhibitory effects on inflammatory responses during the progression of ocular inflammation, encompassing various types such as infectious, non-infectious, or autoimmune, and complications generated by oxidative stress [35]. However, there is now a dearth of research investigating the possible impacts of providing EGCG especially for the treatment of bacterial keratitis, particularly in instances involving P. aeruginosa, to the best of the author's knowledge.
In the dry eye syndrome rabbit model, the administration of gelatine-EGCG nanoparticles with hyaluronic acid surface ornamentation twice daily, resulted in a reduction in the levels of TNF-α, IL-6, and IL-8, as determined by ELISA. These levels were comparable to those observed in the control group, which consisted of rabbits with normal corneas [34, 35]. The anti-quorum sensing and anti-infective activity of EGCG against P. aeruginosa was assessed in an in vitro study conducted by Hao et al. (2021). The EGCG compound had notable inhibitory effects on many biological processes, including the formation of biofilm, protease and elastase activity, as well as swimming and swarming motility [41]. The research conducted by Ruban et al. (2017) investigated the effects of voriconazole and topical EGCG on animal models with fungal keratitis caused by Fusarium solani. The study found that on the 15th day of treatment, there was a significant reduction in the mRNA transcription levels of TNF-α and IL-1β in the group receiving both voriconazole and EGCG, compared to the groups receiving only voriconazole, only EGCG, and the control group receiving saline only. This suggests that the combination therapy of voriconazole and EGCG led to a comprehensive resolution of inflammation and oxidative stress [42].
Pseudomonas keratitis animal model
Current scientific investigations are focused on comprehending the underlying processes of pathogenesis and inflammatory responses associated with bacterial infections in the eye, particularly in regions that possess "immune-privileged" status. The ocular manifestations of infectious diseases are influenced by both bacterial colonization and virulence factors, as well as the host's immune responses to these infections. Several animal species, including rats and rabbits, are utilized as models for studying bacterial keratitis. There exist several distinctions between human and animal eyes, encompassing unique anatomical characteristics, distinct tissue composition, and diverse functionalities of ocular components in each species. The corneal diameter of humans measures approximately 11 mm, whereas rats have corneal diameters ranging from 2.2 to 3.5 mm, and rabbits possess corneas measuring approximately 13 mm. The corneal thickness is greater in humans compared to that in rats and rabbits. Rabbits possess nictitating membranes, sometimes known as third eyelids, which are absent in humans and rats. There exist variations in corneal collagen and corneal epithelial cells among mice, rabbits, and humans, resulting in diverse corneal responses to the pathogens presence. The thickness of Descemet and Bowman membranes in rats and rabbits is comparatively lesser than that observed in humans. The cornea of rats exhibits a higher proportion of corneal epithelial cells relative to the stroma when compared to humans. In addition, rats possess a greater number of layers in the corneal epithelium [43].
Several techniques have been employed to induce keratitis in rat’s models. The prevailing approach involves employing scratching techniques to abrade the corneal surface, followed by the application of solutions containing the bacterium P. aeruginosa for detoxification purposes. Nevertheless, certain research employing the solution utilize varying levels of germ concentrations. There exist multiple techniques for inducing keratitis, such as the utilization of scratching, subsequent application of bacterial droplets onto the ocular surface, and the inoculation via intrastromal injection of bacteria. The method for inducing bacterial keratitis, as described in previous studies, involves the application of a 5 μl suspension of P. aeruginosa containing 2×106 CFUs onto the cornea of a rat's eye. This is achieved by initial creation of a 3 mm diameter epithelial layer scratch using a scalpel, followed by creating a wound on the stroma using a sterile 25-gauge needle. At the 12-hour mark following inoculation, opacity was observed in the corneas. This opacity continued to increase at the 24-hour mark. By the 48-hour mark, all corneas exhibited abscess formation. Furthermore, by the 72-hour mark, all corneas displayed abscess formation accompanied with corneal thinning. [43, 44].
Conclusion
The potential anti-inflammatory and antioxidant attributes of Epigallocatechin gallate (EGCG) found in Camellia sinensis extract offer a promising avenue to explore EGCG as an adjuvant therapy in the treatment of bacterial keratitis.
Limitations
This literature review consolidates the existing knowledge regarding the EGCG impact on keratitis treatment, particularly as an anti-inflammatory adjuvant therapy. Nevertheless, there remain unanswered questions that warrant attention in forthcoming studies, particularly in terms of comprehending the distinct expression and significance of TNF-α and IL-10. Further investigation is necessary to determine the appropriate dosages of EGCG for its anti-inflammatory effects in the treatment of pseudomonas keratitis, as well as the optimal composition of the vehicle for topical administration.
Acknowledgements
The authors extend their gratitude to Dr. Nurwasis, dr., Sp.M(K) from the Ophthalmology Department at Universitas Airlangga's Faculty of Medicine for providing support in developing the research concept.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
ORCID
Ismi Zuhria
https://orcid.org/0000-0003-2102-7782
Fathimah Fariztah Sukainah Nur
https://orcid.org/0009-0004-1539-1945
Urolita Tarosa Yodia
https://orcid.org/0009-0009-7297-0536
Karima Annisa
https://orcid.org/0009-0004-0183-4362
Lutfi Delfitri
https://orcid.org/0000-0002-5899-818X
HOW TO CITE THIS ARTICLE
Fariztah Sukainah Nur Fathimah, Tarosa Yodia Urolita, Annisa Karima, Ismi Zuhria*, Delfitri Lutfi. Anti-Inflammatory Effect of Epigallocatechin Gallate on Tumor Necrosis Factor-Alpha and Interleukin-10 Expression in Pseudomonas Aeruginosa-Induced Keratitis. J. Med. Chem. Sci., 2024, 7(1) 250-261.