Document Type : Original Article
Authors
- Tarini Mullick 1
- Sushruta S Hakkimane 2
- Kishore Ginjupalli 3
- Swathi Pai 4
- Bharath Raja Guru 5
- Krishnaraj Somayaji 1
1 Department of Conservative Dentistry and Endodontics, Manipal College of Dental Sciences, Manipal academy of higher education, Manipal, India
2 Department of Biotechnology, Manipal Institute of Technology Bengaluru, MAHE, Manipal, India
3 Department of Dental Materials, Manipal College of Dental Sciences, MAHE, Manipal, India
4 Department of Conservative Dentistry and Endodontics, KVG Dental College, Sullia, India
5 Department of Biotechnology, Manipal Institute of Technology Manipal, MAHE, Manipal, India
Abstract
Partial or an incomplete penetration of bonding agent into the demineralized dentin can leave the denuded collagen fibres susceptible to degradation by photolytic enzymes. It is well known that the demineralized dentin and its collagen network is susceptible to degradation with time which can significantly affect the bond strength between root canal and sealer materials. The current study attempts assess the protective effect of proanthocyanidin (PA) loaded PLGA nanoparticles in minimizing the deterioration of demineralized dentin over a period. This is realized by determining/evaluating the push out bond strength between a glass fibre post cemented to root canal using resin luting cement. Ninety human mandibular premolars with single root canals were randomly divided into nine groups (n=10) after endodontic treatment and post space preparation. The root canal dentin of the control group was left unconditioned and the experimental groups i.e. proanthocyanidin and PA loaded PLGA nanoparticles (PA-NP) were conditioned respectively with the test solutions. The glass fibre posts (GFP) were cemented into the root canal with dual cure resin cement (RC). The push out bond strength (POBS) was measured at 30, 60, and 90 days. The mode of failure was evaluated with the help of stereomicroscope. The POBS were statistically analysed and evaluated with the help of paired t-test, ANOVA test and post hoc Bonferroni test at a confidence interval of 95% (α = 0.05). Unloaded NP's average particle size was found to be 237 16 nm, and its zeta potential was -54±4 mV. There was a statistically significant difference between the mean POBS at all time intervals among the groups tested. PA-NP showed a statistically significant higher bond strength compared to control at all time intervals, while it showed significantly better POBS compared to PA at 180 days. All the experimental groups showed higher POBS at all intervals of time compared to control group. Application of natural cross-linkers such as PA improves POBS of GFP bonded to root canal dentin. PA loaded PLGA nanoparticles (PA-NP) as a conditioning liquid prior to root canal cementation not only improved POBS significantly, but also reduced the time dependent reduction in the bond strength.
Graphical Abstract
Keywords
Introduction
Restoration of the tooth following endodontic treatment is an important factor that determines the long-term success of an endodontically treated tooth. When inadequate tooth structure remains to provide sufficient retention, the post-endodontic restoration can be challenging for the clinician. Endodontic posts are often used to aid in the retention of a core, laying the foundation for the indirect restoration following endodontic treatment [1]. Prefabricated fibre posts not only save chairside time but also offer superior success compared to metal owing to their low elastic modulus that enables them to show similar mechanical response to that of tooth structure [2-4]. The bond strength of intracanal restoration to dentin is crucial to its retention. When a failure occurs in this interface, the risk of failure of the tooth or the restoration rises [5]. Studies have demonstrated that bonds in the hybrid layer with modern hydrophilic dentin bonding agents weaken over time due to hydrolytic degeneration of collagen by proteolytic enzymes [6].
It is believed that the adhesion related to phase of resin and dentin is weak in fibre post luting. The destruction of collagen fibrils exposed to partially infiltrated hybrid layers is likely what causes the loss of integrity of resin-dentin bonds over a longer period. This process is likely facilitated by the matrix metalloproteinases (MMPs) [7], which are found in radicular dentin. Proanthocyanidins (PA) are bioflavonoids, readily extracted from grape seeds with common solvents.
They are not toxic and their cross-linking action on collagen fibres helps in stabilization of the collagen matrix. They possess the ability to bind to proteins that are rich in proline [8] such as collagen. PA is used in adhesive dentistry for its ability to cross-link collagen and improve bonding. The greater surface area was provided using nano sized particles of a material that enabled the reduction of the amount required for a specific task. Polymeric nanoparticles have been used to control the release of drugs [9, 10]. A sustained release delivery system enables the drug to have a prolonged therapeutic effect by its slow release over a period following the administration of a single dose [11].
Poly (D-L lactide-coglycolide) acid (PLGA) has been used for the nanoparticles preparation with the capacity to contain therapeutic agents due to biocompatibility, biodegradability, and sustained-release properties [12]. Collagen cross-linkers with a property of sustained-release are desirable which can possibly help countering the process of hydrolytic degradation of the interface between the root canal and the filling material [13].
Such materials can significantly improve the long-term success of post and core restoration, by delaying the degradation of the collagen matrix. PLGA NPs offer an attractive option as carriers for such cross-linkers, such as PAs. Fawzy et al. (2017) showed results supporting the assumption that greater the PA loading into the PLGA NPs, more the drug will be gradually released to cross-link with the collagen, enhancing its biodegradation resistance [13]. Despite the beneficial effects of PA on improving the biodegradation resistance, their use on root canal conditioning materials prior to post cementation and its effect on push out bond strength over a period has not been investigated.
The purpose of this study was to formulate, characterize, and evaluate the POBS of Proanthocyanidin loaded PLGA nanoparticles on GFB when bonded with resin cement to root dentin in human teeth.
Thus, the aim of the present study was to evaluate the POBS of GFP bonded with resin cement (RC) to the root canal dentin conditioned with proanthocyanidin nanoparticles (PA-NPs) at different time intervals.
The assumption made in the null hypothesis stated that root canal dentin conditioning with proanthocyanidin-loaded PLGA nanoparticles will not influence the POBS of GFP bonded with resin luting agent.
Materials and Methods
Proanthocyanidin nanoparticles
Preparation of proanthocyanidin solution
Proanthocyanidin was derived from commercially available grape seed extract powder (Nutrija, India) having a purity of over 95%.
PA solution and PA-NP solution was prepared by weighing known number of samples and dissolved/dispersed in water keeping final PA concentration as 10%.
Preparation of proanthocyanidin encapsulated nanoparticles
The solvent evaporation water-oil-water (double-emulsion) process is used to create the NPs. At 4 °C, for 3-4 hours, 80 mg of PLGA was solubilized in 3 ml of dichloromethane in a 15 ml tube. About 40 mg of PA was solubilized in 1:9 of Ethyl alcohol and water. This aqueous solution was emulsified in 3 ml of dichloromethane containing polymer 1:1 by sonication. Primary emulsion was poured into 8 ml of water containing 2% polyvinyl alcohol in a 50 ml glass beaker to form water in oil in water emulsion and is sonicated for 7 to 8 minutes at 40-45% amplitude using an ice bath. Rota evaporation was done to the emulsion obtained to remove organic solvent. Volume was made up to 25 ml with distilled water and centrifuged at 13,000 rpm at 4 °C for 30 min. Particles thus obtained were re-suspended in 15 ml distilled water and sonicated for 30 seconds at 40% amplitude. Centrifugation was done at the same conditions to wash away the PVA. The NPs obtained as a pellet were dispersed in 10 ml of distilled water by sonication for 30 seconds. To create powder form of the NPs, this NPs dispersion was lyophilized for 48 hours after being frozen overnight at -80 ᵒC. Control NPs were prepared in a similar way using chloroform and 2% PVA solution, without PA. Obtained PA-NPs were stored at -20 °C and used for further studies [14].
Calibration graph and proanthocyanidin encapsulation
To make the stock solution of PA, 10 mg of PA was dissolved in 10 ml of methanol, to achieve 1 mg/ml as the final concentration. Seven varying concentrations, staring from 10 μg/ml up to 200 μg/ml were used to plot the calibration curve graph. The highest absorbance was taken by Bio Photometer Eppendorf at 280 nm. The known amount of PLGA-PA NP was weighed in 2 ml vials in triplicates. To release the PA, one ml of methanol was added to each vial, and the vials were shaken on a rocker for two days. After centrifuging the vials for 20 minutes at 10,000 rpm, the supernatant was measured at 280 nm.
Assessment of zeta potential and size of particle
To measure these parameters, Horiba Scientific SZ-100 (Japan) was used by DLS method and were sonicated (Grant Instruments-UK) about 45 seconds in cold condition. Lyophilized NP samples were re-dispersed with Millipore water. The experiments were performed at 25 °C. The NPs' zeta potential was assessed using the instrument's electrophoretic cell. The average values were noted.
Morphology by scanning electron microscopy
Field emission microscopy (FeSEM) (Carl-Zeiss AG Germany, Gemini SEM 300 CRF, NITK) was done to characterize NPs shape and morphology at a magnification of 50.00 kX with scale bar of 400 nm. Dry form of samples was put on the specimen-stub after the gold sputter coating and pictures were captured.
Proanthocyanidin nanoparticles release study
We investigated PLGA NPs loaded with PA to study the in-vitro release pattern. A buffer with a pH of 7.4 was used to disperse 3 mg of NPs in triplicates using 2 ml vials. To simulate the required condition, a rocker shaker with required temperature was maintained. Vials were incubated in shaking position at 37 °C. Initial time was noted and at time intervals, the supernatant was collected from each vial by ultracentrifuging at 10,000 rpm for 30 minutes. Supernatant was analysed using biophotometer and the readings were noted. The NP pellets were re-dispersed in 7.4 pH buffer in a specified volume, and the incubation was maintained for 20 days in a rotary shaker. The total amount of proanthocyanidin was released from the NPs during this experiment, expressed regarding time in days and hours.
Preparation of tooth samples for Fourier Transform Infrared Spectroscopy (FTIR) analysis
Dentin discs were obtained from the middle third of single rooted teeth. Using a diamond disc, dentin slices of 1 mm thickness were obtained and polished to a smooth surface. The dentin slices were treated with 37% phosphoric acid and dried with absorbent paper to prevent excessive drying, following which FTIR was recorded. The samples were divided into three groups. The control group was left untreated, PA and PA-NP groups were conditioned with the respective solutions for one minute. The samples were then stored in collagenase Type I solution for a period of 30 days at 37 °C. FTIR was repeated for these samples to check for degradation.
Push out bond strength
Tooth preparation
After approval from Ethics Committee (IEC/758/2018), ninety single-rooted mandibular premolar teeth were selected for the study, with a sample size of 10 teeth for each group (n=10).
Teeth with straight roots and fully formed apex that were extracted for periodontal reasons were included in the study. Teeth with fractures, curvatures in roots, root caries, and resorption were excluded from the selection. Tissue fragments and calcified debris were removed from the surface of the teeth and autoclaved. They were decoronated with a diamond disc and the root length of 12 mm was standardized for all the teeth. Pulp tissue was extirpated with a barbed broach. Working length for all the teeth was determined by visualizing the tip of the file at the apex under loupes and reducing 1 mm from the determined length. Biomechanical preparation was performed by K-files and ProTaper Gold rotary files (Dentsply Tulsa Dental Specialties, Tulsa, OK), enlarging the canal sequentially up till the size F2. Alternating irrigation was performed with 2.5% Sodium Hypochlorite (Sigma-Aldrich) and 17% EDTA (Sigma-Aldrich) at 5 ml/min each for one minute between each file size and normal saline at 5 ml/min was used as the final rinse. The teeth were embedded in acrylic cylinders, such that the long axis of the tooth was parallel to that of the long axis of the cylinder. Post space was created with Peeso Reamers (MANI, Japan) until Peeso size 3 till a post space length of 8 mm. The fit of the GFP size #1 was checked at this stage. The final smear layer was removed by irrigating the canal with 17% EDTA at 5 ml/min for one minute followed by rinsing with saline. The root canal dentin walls were etched with 37% Phosphoric acid (Eco-Etch Ivoclar Vivadent, Schaan, Liechtenstein, USA) for 15 seconds. At this point, the prepared samples were randomly divided into different groups as Table 1.
Root canal conditioning and bonding procedures
While the control group was left unconditioned, 1 ml of test solutions effectively containing 10% solution of PA was introduced in the canal of the experimental groups using a syringe and needle for 30 seconds. The solution was activated for five seconds with the help of an endoactivator (Dentsply, Maillefer, Tulsa, OK, USA). Another one ml of the solution was irrigated into the canal for 30 seconds to replenish the solution followed by activation with endoactivator.
The remaining solution was then dried with absorbent points. An adhesive was applied on the root canal dentin wall in all the groups and cured for 20 seconds. The GFP was conditioned with 10% Hydroflouric Acid for 60s succeeded by application of silane coupling agent. Dual Cure resin cement (Core X Flow- RC (Dentsply De Trey, Konstanz, Germany) was applied to the root canal’s walls and the GFP was placed in the canal and was cured for 40 seconds. The specimens were then stored in distilled water for 30, 60, or 180 days. The specimens were then sectioned horizontally in the middle third to form 2 mm thick transverse sections using a Low-Speed Diamond Wheel Saw. The sections were subjected to POBS testing using a Universal Testing Machine (UTM) (Model 3366, Instron, USA) at a cross head speed of 1 mm/min. The plunger of the UTM was customized to have a cylindrical thickness of 0.9 mm, i. e. 85% of post diameter (1.1 mm), as suggested by Chen et al. (2013) [15].
POBS was calculated using the formula- POBS= Peak Force/Area of bonded surface.
A=2πrh
Where, ‘r’ is the radius of the post and ‘h’ is the thickness of the section.
The mode of failure was analysed for all the samples under the stereomicroscope.
Following are the categories for failure modes: (Freitas et al., 2019)
I: The following adhesive failures exist within the RC and the root canal,
II: Among the RC and the fibre post,
III: Failure of cohesion in the RC,
IV: Cohesive fibre-post failure,
V: Root dentin cohesive failure, and
VI: Combined failure.
Table 1. Various groups and time at which Push out strength was analyzed
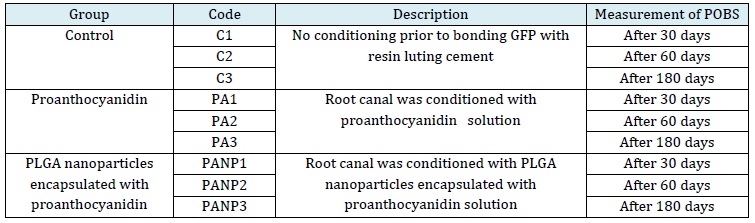
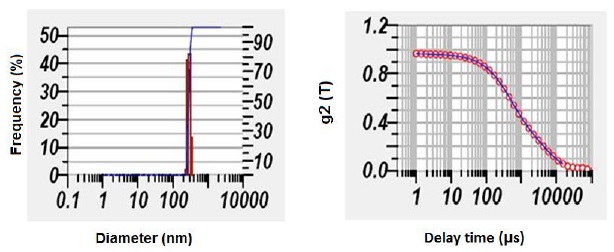
Figure 1: Size distribution of PLGA PA nanoparticles
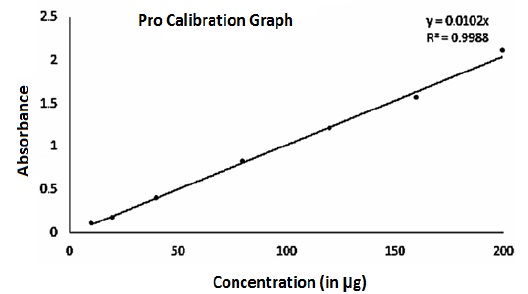
Figure 2: Pro-calibration graph representing encapsulation efficiency
Results and Discussion
The data was analysed by Paired t-test for intra-group analysis and by ANOVA test and post hoc Bonferroni test for inter-group analysis using Statistical Package for Social Sciences (SPSS) software version 20 (IBM SPASS statistics [IBM corp. released 2011]. The level of significance (p-value) was calculated to be less than or equal to 0.05.
Preparation of proanthocyanidin encapsulated nanoparticles
In this study, the yield for the solvent evaporation procedure was between 50 and 60 percent for the PA-NP formulation batches.
Evaluation of size distribution and zeta
According to the particle size distribution graph, the particle mean size of unloaded NP was observed to be 237 ± 16 nm, and the zeta was −54 ± 4 mV. Whereas PLGA NPs loaded with PA was found to be 295 ± 11 nm with a zeta potential −45 ± 3 mV confirm stability of the nanoparticles (Figure 1).
Encapsulation efficiency of nanoparticles
The peak area ratio of the drug was taken into consideration while creating the graph. The standard curve was observed to be straight in the measured series of concentrations. With an R2 value of 0.9968, in the graph, equation curve noted was y = 0.0102x, where "y" represents peak area whereas "x" for the drug concentration (Figure 2). The PA quantity (in micrograms) present in one mg of NPs is taken to determine drug entrapment. The quantity of drug entrapped in customized PLGA-PA-NPs was 158±4 μg/mg.
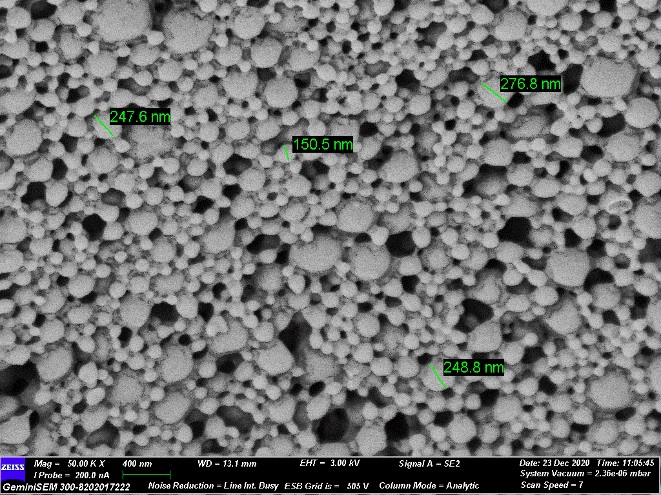
Figure 3: SEM image showing surface morphology and shape of the PA-NP
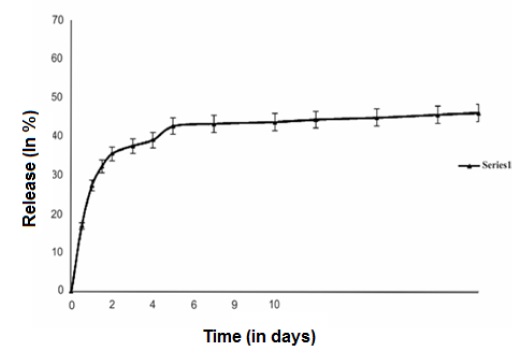
Figure 4: Release profile of PA
Characterization of nanoparticles by SEM
SEM pictures (400 nm scale) revealed PA loaded NPs (Figure 3) were fine and there were no significant cracks, perforations, or other surface defects visible in the NPs. Formulated particles were in spherical shape and had relatively uniform size distribution (Figure 3).
Proanthocyanidin nanoparticles release study
The collective total amount of drug released in percentage terms with respect to time in days is determined as vitro drug release.
The in vitro release profile showed a long and continuous release phase as the drug trapped in the NPs was released into the medium after an initial modest burst release phase (17%) (Figure 4), and 46% of the drug was liberated after 20 days.
POBS of glass fibre post
Figure 5 demonstrates the graphical representation of comparison of POBS within the group between the time intervals (30, 60, and 180 days). The main aim of the present study was to evaluate the effect of conditioning of root canals with PA and PA NP along with PLGA after the post space preparation on the POBS of the fiber post at 30, 60, and 180 days.
From Figure S1A (Supporting information), it was observed that the POBS of control specimens at 30-day interval was 3.32 MPa. The POBS of root canal conditioned with PA after post space preparation resulted in a POBS of 5.29 MPa which is significantly higher compared to control group (p<0.001). Similarly, the use of PA nanoparticles along with PLGA resulted in a significantly higher POBS of 6.45 MPa (p<0.001). The values derived after root canal conditioning with PA on the POBS at 60-days interval is presented in Figure S1B (Supporting information). The POBS of control group was observed to be 2.54 MPa whereas it was 3.98 MPa for PA group and 6.42 MPa for PA-NP group. The observed POBS values are significantly higher compared to control group (p<0.001). The POBS measured at 180 days interval for the control group was 1.49, while for PA and PA-NP, was 3.33 and 5.33, respectively. As observed with both 30 and 60-day intervals, both PA and PA-NP groups showed significantly higher strength compared to control group (P<0.01). It was also observed from post-hoc analysis of the results using Mann-Whitney test that pushout bond strength values are significantly different among the groups except between control and PA group at 60 days (p=0.049), and at 30 days (p=0.049), and at 60 days (p=0.019) between PA and PA-NP groups.
Bond strength
From the results, it was also observed that there is a progressive reduction in the POBS among all the groups. However, the extent of reduction of the POBS seems to be higher in control group compared to PA and PA-NP. In the control group, the amount of POBS reduction was observed to be about 30% reduction at the end of six months. PA group exhibited reduction in the bond.
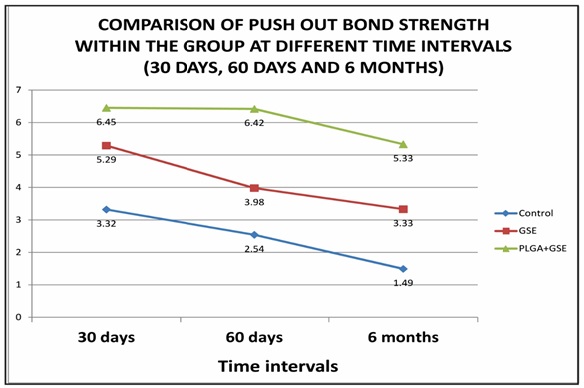
Figure 5: Graphical representation of comparison of POBS within the group between the time intervals (30, 60, and 180 days)
Strength of 22% and 36% at 60-days and 6 months, respectively. PA-NP group showed a reduction in the POBS of 8% and 17% at the same time intervals indicating that both PA and PA-NP groups not only exhibited higher POBS, but also showed lower degradation in the bond strength. Among PA and PA-NP groups, the percentage reduction in the bond strength was observed to be less with PA-NP groups.
FT-IR results
FT-IR analysis exhibits the chemical analysis of collagen fibrils. The spectra of crosslinked collagen were obtained using Shimadzu FT-IR spectrometer at a resolution of 4 cm-1 by applying a thin film of sample on sensing window of ATR. In the control sample, before and after degradation spectrum showed an increase in the degradation in Figure S2A and S2B (Supporting information). However, root canal dentin that is conditioned with PA or PLGA nanoparticles encapsulated with PA, there was not much increase in degradation as indicated by Amide I (1635 cm-1) and Amide II (1548 cm-1) bands in the IR spectrum. Comparatively, the efficacy in stabilizing dentin collagen against enzymatic degradation was observed (Figure S2C, Supporting information)
Stereomicroscopic analysis of the mode of failure revealed that the most common mode of failure was mixed, followed by an adhesive failure between post and cement (Figure 6).
Etch and rinse adhesives require the demineralization of dentin followed by application of an adhesive to create a hybrid layer [16]. Furthermore, the use of etch and rinse adhesives can result in the activation of endogenous matrix metalloproteinases (MMPs) and cysteine cathepsins (CTs) present in the dentin, triggering hybrid layer degradation.
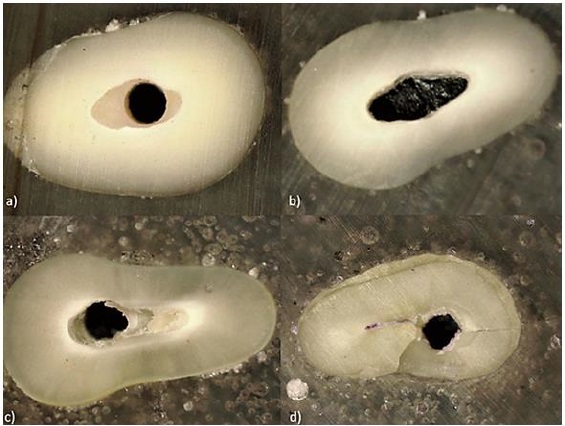
Figure 6. (a) Adhesive (post and cement), (b) adhesive (cement and dentin), (c) mixed, and (d) cohesive-within dentin
According to [17], water-rich zones are usually sparse immediately after bonding; they increase in size with time [18], leaving exposed collagen fibrils susceptible to time-related hydrolytic degradation. The mechanical properties of dentin significantly decrease after demineralization. The biomodification of demineralized dentin contributes to its mechanical properties via nonenzymatic cross-linking of exposed collagen fibres, strengthening un-infiltrated areas of exposed collagen, increasing the elastic modulus of dentin and inhibiting collagenolytic activity [19]. PAs induce exogenous cross-links, inactivating proteolytic enzymes by a nonspecific mechanism and decrease collagenase absorption, increasing the density of collagen matrix. This is indicated by the decreased swelling ratio of demineralized dentin, thus increasing the dentin resistance against enzyme-based deterioration [20]. While other agents like chlorhexidine form reversible electrostatic bonds and PA crosslinks through covalent bonds maintain their stability over time.
PLGA is a safe, versatile polymer which is completely biodegradable. Their degradation rates can be adjusted by varying the lactide to glycolide ratio. Most NPs prepared from PLGA are less than 500 nm in size, with a spherical shape and a smooth surface. They release the drug with an initial burst followed by a sustained release [21].
Fawzy et al. in 2017 [13] investigated the effect of PA delivery to demineralized dentin via loading into PLGA NPs and found that the treatment improved the collagen structural resistance along with the biomechanical and biochemical stability of demineralized dentin. In this study, PAs were used from a commercially available health supplement of grape seed extract (Nutrija, India) containing 95% PA, similar in composition to Fawzy et al. The PLGA NPs loaded with PA were successfully synthesized with average particle size of 295 nm, with particles sizing as low as 150 nm, with a PLGA ratio of 75:25. SEM revealed spherical shaped particles, confirming the NP synthesis. Based on the previous reports, concentrations ranging from 3.75% to 15% of PAs have been used to enhance the biodegradation resistance of dentin, thus an effective drug concentration of 10% PA was taken into consideration in this experiment for both the experimental solutions. An encapsulation efficiency of 158±4 μg/mg was achieved in the PLGA NPs loaded with PA and the average zeta potential was -45 ± 3 mV indicating good loading capacity and stability.
PLGA as drug carriers, release the drug with an initial burst followed by a sustained release [22]. In our findings, an initial burst of 17% at 2-3 days was observed, followed by 46% of drug release at 20 days, this is in partial agreement to the previous study, where the initial burst of drug release was seen followed by a gradual release of the drug over 28 days, due to monomer ratio of PLGA, used giving a slow and sustained release. To assess the PA efficacy in preventing hydrolytic degradation of collagen network of the dentin, FTIR spectroscopy was carried out. From the FTIR spectroscopy, it was observed that the use of either PA or PA in the form of PLGA NP ensured integrity of tooth structure as evidence from the transmission spectra recorded. There is a distinct difference in the FTIR spectrum of control, acid etched, and those subjected to collagenase treatment. However, the FTIR spectra of the dentin specimen subjected to PA or PLGA+PA was similar to that observed with control specimens indicating the protective nature of PA or PLGA+PA against the hydrolytic degradation of collagen network [23].
Thus, to evaluate the significance of this finding in relation to the POBS of GFPs, our study investigated the effect of conditioning the root canal dentin with PA and PLGA NPs loaded with PA (PA-NP). The null hypothesis was rejected, as there was a statistical difference in the groups with PA-NP showing the maximum POBS at all time intervals of 30, 60, and 180 days. Since it is well-established that the initial POBSs are within the clinically acceptable limits and the decrease in POBS due to hydrolytic degradation occurs over time, the time intervals of 30, 60, and 180 days were selected. Testing the bond strength at time intervals of 30, 60, and 180 days permitted the effect evaluation of the slow sustained release by loading the drug on PA-NPs as compared to the direct application of the PA on the bond strength of the RC to root canal dentin. In bonded restorations, nano leakages can occur due to a gap of 20-100 nm width in the hybrid layer. These spaces are small enough to permit the penetration of water leading to the deterioration of the resin-dentin bonds over time.
Therefore, the NP size in this study was under dv300 nm to permit adequate penetration into the demineralized collagen matrix in dentin. The bond strength between the root canal filling material and dentin decreases cervico-apically [24]. This may be attributed to various factors such as the sparse dentinal tubule configuration in the apical areas of the canal, tubular sclerosis, the C factor [25], the limited access, and visualization of the apical portion of the canal, and restrictions in the flow of the cement into this area. This can negatively affect the bond strength for the middle and apical thirds of the root canal. Collagen cross-linkers can play a vital role in stabilizing the exposed collagen entrapped in the water spaces in the hybrid layer in the lower thirds. Therefore, the middle third was selected in this study to see the effect of sustained release of PA as a cross-linking agent on the clinical performance of fibre posts. The specimen preparation in this study was performed in a similar experimental set up as proposed by previous study [26]. According to Mazzoni et al. [27], the releasing and activation of proteolytic enzymes detained within the mineralized dentin will be made easier by the total etch adhesives. Human odontoblasts are stimulated to produce MMP-2 because of the adhesive being applied to acid-etched dentin. This MMP-2 may then move through the dentinal tubules to the hybrid layer, where it may exert greater effect. As PAs are known to inhibit MMPs and cross-link collagen, they were used to condition the demineralized dentin prior to adhesive application. Due to the presence of a high number of endogenous MMPs present in dentin activated by etch and rinse procedures, distilled water is an adequate storage medium [28].
Results of the present study indicate that the conditioning with PA before adhesive application increased the POBS of the GFPs at 30, 60, and 180 days’ time intervals compared to control. These results agree with Shen et al. [29], where the POBSs were higher in groups conditioned with PAs as compared to control. This could be attributed to the increase in the mechanical properties of the un-infiltrated collagen matrix by the cross-linkages and the inhibition of proteolytic enzymes provided by PAs.
However, Alonso et al. demonstrated that PA maintained the POBSs of fibre posts over the period following an initial drop [30].
The overall lower POBSs found in this study could be attributed to the use of circular cross-section posts in oval canals of mandibular premolars which results in less-than-ideal POBSs [31]. This may be due to the incomplete adaptation of the post against the canal walls. In addition, it results in increased RC thickness which can have a negative impact on the POBS. This decrease in POBSs within the groups may be explained by the degradation of the resin tags in the hybrid layer by esterase enzymes which degrade the ester bonds in HEMA [32, 33]. The possible decrease in the sustained release of PA from PA-NP may also affect the results. Present experiment agrees with existing literature which suggests that the application of cross-linkers decelerate the hybrid layer degradation when compared to unconditioned dentin. Presence of NPs may have helped in better penetration of the cross-linking drug to the basal layers of the hybrid layer, resulting in higher POBSs. At 60 and 180 days, it showed a higher POBSs indicating the positive effect of the sustained release of PA on the POBS of fibre posts. This finding is supported by Fawzy et al., who demonstrated that the sustained release of PA contributed to the biodegradation resistance of the exposed collagen fibrils at the base of hybrid layer. Stereomicroscopic analysis revealed that the most common mode of failure was mixed and might be attributed to the variable cross-section of mandibular premolars which would favour the retention of RC. The results should be interpreted with caution, as the POBSs in the present study may not be precise compared to those in other studies. Despite the best efforts to maintain the standard of sources used, there may be a variation in the commercially available grape seed extract, thus leaving room for variability in the quality of the PA employed. There may also be variations in the composition and size of tooth specimens used for the experiment. While the results of this study are promising in the aspects of sustained release in PAs, future studies evaluating the long-term POBSs of fibre posts are essential.
Conclusion
The POBS between root canal dentin and GFP luted with resin luting cements decrease with time due to hydrolytic degradation of hybrid layer. Root canal conditioning either with PA or PLGA nanoparticles prior to GFP cementation to the root canal significantly delays the degradation of hybrid layer. Improved bio resistance of hybrid of the root canal dentin treated with PA or PLGA nanoparticles encapsulated with PA resulted in superior pushout bond strengths. The use of PA as encapsulated in PLGA nanoparticles significantly delayed the degradation of root canal dentin and thus resulted in superior push out bond strengths. However, further studies are encouraged to evaluate the long-term outcomes of this sustained release and its applications in other spheres of restorative dentistry.
Acknowledgements
The authors would like to thank Manipal academy of higher education, India for their support in encouraging.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ Contributions
Designing: Krishnaraj Somayaji, Tarini Mullick, Sushrutha Hakkimane, Bharath Raj Guru, idea making: Tarini; conducting: Tarini, data analysis: Kishore Ginjupalli, Krishnaraj, and analysing and writing: Tarini, Swathi pai, and Sushrutha.
ORCID
Tarini Mullick
htpps://orcid.org/0009-0002-9866-8553
Sushruta S Hakkimane
https://orcid.org/0000-0002-3675-7422
Kishore Ginjupalli
https://orcid.org/0000-0002-1824-2054
Swathi Pai
https://orcid.org/0000-0002-3909-1507
Bharath Raja Guru
https://orcid.org/0000-0003-2542-7740
Krishnaraj Somayaji
htpps://orcid.org/0000-0002-5116-4167
Supporting Information
Copies of graphical representation of comparison of POBS and FT-IR spectra of synthesized compounds.
HOW TO CITE THIS ARTICLE
Tarini Mullick, Sushruta S Hakkimane, Kishore Ginjupalli, Swathi Pai, Bharath Raja Guru, Krishnaraj Somayaji. Development, Characterization, and Influence of Proanthocyanidin Loaded PLGA Manoparticles on Push out Bond Strength of Glass Fibre Posts Bonded with Resin Cement to Root Dentin: In Vitro Study. J. Med. Chem. Sci., 2024, 7(1) 189-202.


