Document Type : Original Article
Authors
1 Department of Pharmacognosy and Medicinal Plants, College of Pharmacy, Mustansiriyah University, Baghdad, Iraq
2 Department of Pharmacognosy and Medicinal plants, Osol Al-Deen University College, Baghdad, Iraq
3 Department of Pharmacology and Toxicology, College of Pharmacy, Mustansiriyah University, Baghdad, Iraq
Abstract
Vitex negundo Linn, known as chaste tree, belongs to Verbenaceae family. It is a woody medicinal tree; an aromatic and flowering-ornamental plant has different ethnobotanical and pharmacological uses. The aims of this study included phytochemical analysis, detection of two flavonoids by TLC, GC/MS analysis of hexane extract, and the antioxidant estimation in leaves of Iraqi Vitex negundo activity. The results showed the important phytochemicals in hydroalcoholic and hexane extracts. In addition, the main constituents obtained by GC MS were Sabinene 3.23% and Pinene 0.87% as monoterpenes, alpha-Farnesene 0.5%, Pregnan-3,11-diol-20-one 1.55%, and alpha-Tocopherol 1.55%. Those Iraqi Vitex negundo L. were considered as an important source for chemical constituents with a significant antioxidant activity at low concentration with IC50 26.7, as compared with ascorbic acid.
Graphical Abstract
Keywords
Main Subjects
Introduction
Vitex negundo Linn, known as chaste tree, belongs to Verbenaceae family. It is a woody medicinal tree, native to the Mediterranean regions, while now it is cultivated all over the world as an aromatic and flowering-ornamental plant. Vitex negundo is considered as one of the common species among the genus (vitex), which consists 250 species such as Vitex negundo, Vitex agnus-castu, Vitex trifolia, Vitex polygama, Vitex leucoxylon Linn, Vitex mollis, and Vitex altissima Linn [1]. Vitex tree is characterized as the beautiful little deciduous a medium sized about 3-4 m in high with leaves have approximately 5 leaflets in a hand shape (palmate) arrangement and rounded fruit, black when ripe, with 3-4 mm, while the flower characterized with the aromatic brilliant color varies from violet-deep purple [2]. Furthermore, an ethnobotanical uses of vitex was mainly to reduce breast tenderness, menstrual cycle pain, and amenorrhea symptoms. Likewise, it is regulating the menstrual cycle, regulate hormones related to fertility, and diuretic effects [3]. Most pharmacological effects of this species were as anti-inflammatory, reduce the level of serum prolactin hyperprolactinemia and mastodyni, anti-oxidant, GIT disturbance, analgesic effects, antimicrobial, and anti-tumor activity [4]. Vitex negundo contains various potentially bioactive phytochemicals such as flavonoids, flavonoids glycosides, phenolic compounds, terpenes, and phyto steroids. Terpenoids are considered as one of the most abundant class of the secondary metabolites in vitex plants. Chemically, they consist of five -carbon isoprene units to form the building blocks of hemiterpenes (C5), monoterpenes (ten carbon atoms), sesquiterpenes (fifteen carbon atoms), diterpenes (twenty carbon atoms), and triterpenes (thirty carbon atoms) [5]. Moreover, previous literatures revealed that V. negundo contains a large number of terpenes in leaves oil such as caryophyllene epoxide, δ-guaiene, and ethyl-hexadecenoate, while in flowers oil contain α-selinene, (E)-nerolidol, carryophyllene epoxide, and germacren-4-ol [6, 7].
Materials and Methods
Plant’s collection
Vitex negundo plant was collected in February 2021 from AL-Zawraa Gardens in Iraq. The authentication of plant was done by Prof. Dr. Ibrahim Salih Abass, in Pharmacognocy Department, College of Pharmacy, Mustansiriyah University, Baghdad, Iraq. The leaves’s part was dried in shade and ground to a fine powder by mechanical grinder.
Chemicals
Ethyl acetate, n-hexane, acetic acid, and chloroform (95-97%) were purchased from Merck (Darmstadt, Germany), while the standard kampferol (≥98%), and apigenin (≥97%) were purchased from Hyperchem Company (China) and DPPH (China).
Extracts preparation
50 g of V.negundo leaves was defatted with hexane by Soxhlet for 12 hours, and then the residue was dried and extracted with 90% methanol. Both extracts were concentrated under the reduced pressure and stored in glass containers for further analysis.
Preliminary phytochemical screening
The hydroalcoholic leaves extract was screened by different chemical tests to investigate the major secondary metabolites in this plant [8-10].
Screening of Flavonoids
Alkali test depends on using ethanolic KOH (2 mL) mixed with 3 ml of alcoholic extract. The yellow color is indicated the flavonoids presence.
Screening of tannins
Few drops of crude extract were mixed with 1% aqueous ferric chloride change of dark green to the blue color can predict the tannin presence.
Screening of saponin
Froth assay was used to screen saponin by shaking distilled water vigorously with 5 mL of crude extract for approximately fifteen minutes. A persistent froth (1 cm) can be indicator for the saponins presence.
Screening of terpenoids
Salkowski test were used as screening of terpenoids confirmed by using 4 ml of the hexane and alcoholic extracts were treated with 4 mL extract was added to 2 mL of chloroform and 2 mL of concentrated sulphuric acid, the positive result gives a reddish- brown color.
Screening of sterols and steroids
Dried alcoholic and hexane extract were treated with 1.5 mL of the equal quantity of acetic anhydride and chloroform according to Liebermann reaction, and then the drops of concentrated H2SO4 was added gradually. The appearance of the green color indicates the sterol presence.
Screening of reducing sugar
Fehling test (solution I and II) was done by the addition of 20 mL of diluted sulphuric acid (H2SO4) to 4 mL of the dried extract in a test tube and boil for 10 min, and then it was cooled and 4 mL of Fehling solution and 10% potassium hydroxide were added, the red to blue precipitate was considered as the positive results.
Screening of alkaloids
Approximately few drops of the crude extract were dissolved individually in few volumes of 1% hydrochloric acid, and then it was filtered. After that, the filtrate was reacted with Mayer's reagent; a positive result for alkaloids was indicated by the presence of white precipitate.
Analytical thin layer chromatography (TLC)
Approximately 2 mg of hydroalcoholic extract were suspended in about 1 mL of absolute methanol, applied on plate of analytical TLC was precoated with a silica gel GF254, and then developed with chloroform-ethyl acetate-acetic acid (5:5:0.1) for detecting the constituents, as compared with the standard.
GC-MS analysis condition
The GC-MS identification was carried out to the hexane extract. Chromatograph analysis was done on Agelint (7820A) USA Gas Chromatography/ Mass Spectrometer (GC-MS) at the Ministry of Industry and Minerals- Ibn Al-Baitar research center-Baghdad. The conditions were as follow: carrier gas was helium column, the split ratio was 2.0, the column was used Agelint HP-5ms Ultra lnleit (30 m × 250 µm × 0.25 µm), 250-300 °C, column temperature, rate 10 °C/min, and the injection volume 1 µl.
Anti-oxidant analysis
In this work, we used 1,1–Diphenyl–2 picryl–hydrazyl; (DPPH assay), using a colorimetric technique defined by Anna Floe gel and coworkers by using a methanolic dilution of DPPH. About 2.95 mL of DPPH solution (approximately 1 Mm DPPH in eighty percent of methanol) was assorted with 0.05 mL of sample or standard (different concentrations ranging from 12.5 to 200 mcg per 1 mL methanol). The assay was completed in triplicate, and the preparing mixture was incubated in the dark condition for about half an hour with aluminum foil paper over it. The absorbance decrease was observed at 517 nm [11, 12].
Result and Discussion
The phytochemical analysis revealed a positive result for the presence flavonoids, tannins, reducing sugar, terpenoids, steroids, and saponin, while negative result were found for alkaloids.
TLC
TLC was applied to detect apigenin and Kaempferol in hydroalcoholic extract, as compared with the standard at 0.5 and 0.7, respectively (Figure 1).
Hexane extract identification by GC/MS
Analysis and determination of bioactive chemical components identified in leaves of Iraqi Vitex negundo hexane extract shows the different components and groups of terpenes and steroids, as displayed in Figure 2. Table 1 presents the major components identified in hexane extract of Vitex negundo leaves. The results reveal the percentage of the main constituents were Sabinene 3.23% and Pinene 0.87% as monoterpenes, alpha-Farnesene 0.5% as a sesquiterpene, in addition to the other important terpenes Pregnan-3,11-diol-20-one 1.55% and alpha-Tocopherol 1.55%.
The GC-Mass spectra of the main known chemical constituents are shown in Figure 3.

Figure 1: TLC analysis for crude extract compared with apigenin (A) and kaempferol (K) reference standard
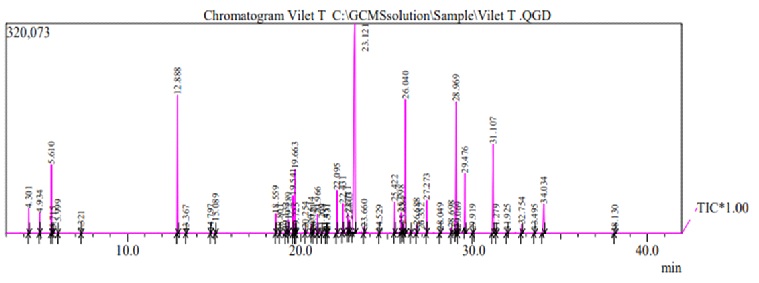
Figure 2: GC/MS chromatogramo of Vitex negundo leaves


Previous studies reported that major components of essential oils in different vitex species consist of sesquiterpene hydrocarbons mainly was caryophyllene which identified in four vitex species which are: V. limonifolia in Thailand [13], V. polygama, V. rufescens, and V. megapotamica in Brazil [14]. Besides caryophylleme, another presented sesquiterpene were δ-cadinene, cis-calamenene, and 6,9-guaiadiene. In addition, the monoterpene hydrocarbons and oxygenated monoterpenes were identified in different regions. The main monoterpene was reported is β-pinene, α-pinene [15], and 1,8-cineole. Moreover, the other terpenoids reported in vitex genus such as abietane-type diterpene, labdane-type diterpenoids, and nor labdane-type diterpenoids [16], triterpenoids oleanane-type, and Oleanolic acid [17].
Antioxidant
Vitex negundo leaves extract indicate a good antioxidant activity when compared with ascorbic acid as shown in Figure 4 and Table 2, 3 and 4. This activity may belong to the presence of a good concentration of various terpenoids or terpenes in Iraqi plant of vitex leaves. The reported previous studies reveled the antioxidant activities of the phytochemical components [18-20].
The results show a significant antioxidant activity at a low concentration with IC50 26.7 as compared with ascorbic acid with IC50=34.74.
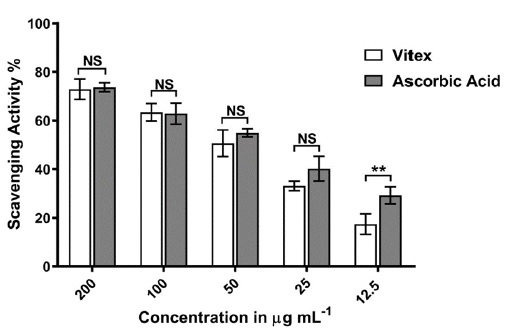
Figure 4: The scavenging activity of Vitex negundo leaves extract and ascorbic acid


Conclusion
Those Iraqi Vitex negundo L. were considered as an important source for chemical constituents with a significant antioxidant activity at low concentration with IC50 26.7, as compared with ascorbic acid.
Acknowledgments
The authors would like to thank Mustansiriyah University (www.uomustansiriyah.edu.iq), Baghdad, Iraq, for its support in the present work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
The author declared that they have no conflict of interest.
ORCID:
Rasha Eldalawy
https://orcid.org/0000-0002-1338-9823
HOW TO CITE THIS ARTICLE
Tahany A. Tawfeeq, Amani A. Tawfeeq, Rasha Eldalawy, Shamis Khaleel Ibraheem. Phytochemical Analysis, GCMS Identification, and Estimation of Antioxidant Activity of Iraqi Vitex negundo L., J. Med. Chem. Sci., 2023, 6(4) 876-883
https://doi.org/10.26655/JMCHEMSCI.2023.4.19
URL: http://www.jmchemsci.com/article_158836.html


