Document Type : Original Article
Authors
- Samaneh Abiri 1
- Alireza Hashemi Shiri 1
- Mohammad Sadegh Sanie Jahromi 1
- Esmaeil Rayatdoost 1
- Hamid Afkhami 1
- Ruhollah Ravanshad 1
- Seyed Ehsan Hosseini 1
- Navid Kalani 1
- Parasta Heidari 2
- Rahim Raoufi 1
1 Research Center for Non Communicable Diseases, Jahrom University of Medical Sciences, Jahrom, Iran
2 Student Research Committee, Bushehr University of Medical Sciences, Bushehr, Iran
Abstract
Background: In traditional Persian medicine, an herbal cold remedy containing Sugarcane, Black Myrobalan, and mastic is mentioned and it was being widely provided by grocery stores. With the advent of the COVID-19 pandemic, the increased desire for this traditional remedy has led to a debate in society. Huge parts of the Iranian population have started using this remedy for the treatment of COVID-19; while no study has evaluated its efficiency and possible side effects. Thus, we aimed to evaluate the effects of Sugarcane, Black Myrobalan, and Mastic herbal medications for COVID-19 patients.
Methods: This was a double-blinded randomized clinical trial study conducted over three months from May 2020 to July 2020 in SARS-COV2 PCR positive patients admitted in the COVID-19 ward of Peymaniyeh Hospital in Jahrom, Iran. Patients with severe COVID-19 infection were not recruited. The intervention group received the routine COVID-19 treatment protocol and the herbal supplement received the combination of black myrobalan and mastic and sugarcane, twice a day (3 g of herbal supplement). Study groups were compared in terms of demographic variables, vital signs, and clinical and laboratory variables for safety and efficiency assessment.
Results: Seventy-two patients with COVID-19, divided into intervention (n=37) and control (n = 35) groups were studied. Intervention and control groups had not any significant difference in terms of baseline characteristics. The time-to-event analysis revealed a statistically significant difference in 4 symptoms of cough, fever, dyspnea, and myalgia (P<0.05). There was no significant difference in averaged O2 vital signs between the two groups (P>0.05). The Control group in comparison to the intervention group had a significantly lower decrease in C-reactive protein during 7 days (P<0.05). Patients in the herbal supplement group were hospitalized for 4.12 days and patients in the control group were hospitalized for 8.37 days (P=0.001). ICU admission and death only happened in 3 (8.6%) patients of the control group.
Conclusion: This study showed that the proposed herbal remedy could be applied as supplementation to conventional management of COVID-19 patients with mild disease, while more research is needed for clinical application of this remedy.
Graphical Abstract
Keywords
Main Subjects
Introduction
COVID-19 is a viral disease that has been responsible for the deaths of large numbers of people around the world in 2020. COVID-19 causes pneumonia, with classic symptoms of fever, cough, dyspnea, and myalgia [1, 2]. It can have so wide range of symptoms, and also damage other organs such as the heart, liver, and kidneys. Some patients eventually die from multiple organ failure, shock, coagulopathies, acute respiratory distress syndrome, heart failure, arrhythmia, and renal failure with different intensities in [3-7]. COVID-19 pandemic has widely affected all aspects of human life, as well as contributing stressful situations in society [8-10], and also management of other health related conditions [11]. During this pandemic, various clinical trials on different molecular candidates [12] and vaccines [13] have been launched to examine different medications, mainly with properties in strengthening the body's immune system, antiviral, and anti-inflammatory properties to prevent cytokine storm [13, 14]. Meanwhile, various studies have shown that traditional herbal medicine could improve the symptoms of COVID-19 [15]. In this regard, Traditional Iranian Medicine (TIM) has potential propositions that could be taken to account as medications to improve COVID-19 symptoms. A notable part of the Iranian society has tended to use a historical cold remedy (combination of Sugarcane, black myrobalan, and mastic) that has been got popular among the people. While some are attributing the source of ingredients of this herbal combination to TIM and some thought to be from the Islamic Medicine; Iranian Health authorities have not confirmed Islamic Medicine officially.
Although traditional medicine has a long history in Iranian society and is being widely used by the public, the public should be informed of the potential risks and benefits of this medicine based on scientific evidence to provide an answer to the current debate. Even if the combination is efficient in the treatment of COVID-19, unofficial providing of such remedy can have potential health risks. As people can purchase this combination without a doctor's prescription, this may delay a doctor's referral and have negative consequences for the treatment of COVID-19. Furthermore, WHO guidelines on good manufacturing practices (GMP) for herbal medicines [16] are not followed by current providers of this combination in grocery stores. Therefore, relying on the safety and potential relevant biological effects, reported in the literature, we designed this randomized clinical trial. The first ingredient, Black myrobalan (Terminalia chebula or black myrobalan), has a wide range of biologically active compounds and was being frequently applied in TIM for the treatment of respiratory tract diseases [17, 18].
Sugarcane (Saccharum officinarum), the next ingredient of mentioned combination has been used extensively in TIM [19]. Its beneficial effects are supported by in vivo/in vitro studies, including antihypertensive, anti-inflammatory, anti-hypertensive, and anti-hepatotoxic activity [20, 21].
Pistacia lentiscus resina (mastics), as an herb used in that combination, has been reported to decrease the symptoms of autoimmune diseases by inhibiting the hyperinflammatory pathways [22]. In this study, we examined the combining of these three plants (Sugarcane, black myrobalan, and mastic) along with the treatment protocol of the Ministry of Health on COVID_19 patients with mild to moderate COVID-19 cases.
Materials and Methods
Study design
The present study is a double-blind, randomized clinical trial in adherence to CONSORT guidelines (Figure 1) that was conducted over three months from April 2020 to June 2020 in patients admitted with a diagnosis of COVID-19 in Peymaniyeh Hospital in Jahrom, Iran.
Ethical considerations
Before entering the patients in this study, the research process was explained and informed consent was obtained from them. Throughout the study, researchers adhered to the principles of the Helsinki Declaration and the confidentiality of patient information. All costs of the project were covered by the researchers and no additional costs were incurred by the patients.

This study was approved by the ethics committee of Jahrom University of Medical Sciences under the ethical code IR.JUMS.REC.1399.003 and was registered in the Iranian registry of clinical trials under the number IRCT20200415047082N1.
Sampling
The study population was patients admitted with a definitive diagnosis of COVID-19 in the wards of Peymanieh Hospital in Jahrom. Sample size assuming standard difference=0.85 and confidence limits of 95% and power = 80% and assuming an equal number of samples in each group using Altman nomogram and taking into account 15% precipitation, 70 subjects were determined. Then, to have an equal chance of being in the intervention group or control group, the samples were randomly assigned to the study groups using a random number table.
Inclusion criteria: Patients admitted with COVID-19 with a definitive diagnosis of PCR test, having age over 18 years old, and not being pregnant or lactating. Patients with definitions of severe COVID-19, as well as severe respiratory distress syndrome, organ failure, and ICU admitted patients were not included in the study. The infectious disease specialist supervised these criteria.
Exclusion criteria: Dissatisfaction with participation in the study, dissatisfaction with continuing herbal supplementation, history of severe cardiovascular disease, severe shortness of breath, uncontrolled diabetes, severe kidney or liver disease or any uncontrolled systemic disease, history of drug abuse, and current anti-psychosis (Figure 1 has shown the flowchart of study sampling).
Intervention
All patients with inclusion criteria at the time of the study, after obtaining written consent and explaining the study conditions, entered the study. Patients participating in the present study were divided into intervention and control groups by tossing coins. Patients were adjusted for age and sex. The treatment protocol in the two groups of intervention and control was as follows. The intervention group received the treatment protocol approved by the Ministry of Health of Iran during the period of hospitalization and the herbal supplement obtained from the combination of black myrobalan and mastic and sugarcane, twice a day. The control group only received the approved treatment protocol. Based on a literature review, the optimal doses with the lowest risk of adverse events were chosen. In the case of sugarcane, according to the American Heart Association (AHA), the permissible daily intake of sugar for women is 6 teaspoons equivalent to 25 g, and for men, 9 teaspoons equivalent to 36 g; in our study, 3 grams of sugarcane per day (1.5 grams BID) was used based on the TIM principles, which was safe based on the AHA principles, too. Black myrobalan extract was used in a dose of the 1 g single dose per day.
In the case of the mastic, a dose of 1 g twice daily has been approved for the treatment of benign gastric ulcers. Furthermore, 1 g of mastic was used twice daily in our study.
Herbal supplement production method
To prepare the desired herbal supplement, sugarcane, mastic, and black myrobalan were purchased from approved herb suppliers. The originality of the plants was confirmed by a botanist. Plants were washed and dried to be powder by shredder considering the sterility. Powders were kept in special packages containing 3000 mg of herbal supplements (0.5-gram black myrobalans, 1 g mastic, 1.5 g sugarcane), which were given to patients evening and night before sleep. Based on the TIM guidance, the medication should be used sublingually and the patient had not to try swallowing or chewing it first but had to allow saliva to be secreted and mixed with it and gradually swallow it. Failure to pay attention to this issue causes nausea in the patient based on TIM. It should be noted that drinking water with this supplement and even up to an hour after taking it was forbidden due to the reduced effectiveness of the drug with water, so we ask the patient to help us in this matter.
Control group
The Control group was planned to receive a placebo and the treatment protocol approved by the Ministry of Health of Iran during the period of hospitalization. Placebo was shape, size, and color-matched with the main supplement in the intervention group. It was made of bran and barley powder (for color matching).
Blinding
Based on the randomization outcome declared by the lead nurse, the administration of herbal supplements in the intervention group, and placebo in the control group were performed by nurses. The researchers and nurses did not realize the randomization results and the type of package provided to the patient. The researcher only provided the supplement and placebo to the head nurse, in the same form of packaging.
Data collection
All patients were compared in terms of demographic variables, vital signs, and clinical and laboratory variables. Demographic characteristics included: age, gender, Body mass index (BMI), history of smoking, and occupation. Critical indicators including temperature, systolic and diastolic blood pressure, heart rate, arterial blood oxygen saturation, and respiration rate were examined and recorded daily in both groups. The averaged vital sign values and daily values were compared. The vital sign of each day was recorded three times a day, including temperature (C), blood pressure (mmHg), pulse rate (beats per minute), respiratory rate (beats per minute), and blood oxygen saturation (percent). Clinical characteristics including symptoms (Cough, Dyspnea, Myalgia, Fatigue, sputum discharge, Rhinorrhea, and Headache) were assessed daily with a designed questionnaire. In this questionnaire, the patient first determines the presence of these symptoms at the beginning of the disease and finally choose one of the options (It got much better, it got better, it didn't change, it got worse, and It got much worse); symptoms tracks were recorded for each of these symptoms. Laboratory indices were performed in the first to seventh days of hospitalization. Laboratory indicators included: aspartate aminotransferase (AST), alanine aminotransferase (ALT), prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), blood urea nitrogen (BUN), creatinine (Cr), white blood cells (WBCs), red blood cells (RBCs), hemoglobin (HB), neutrophil, lymphocyte, and monocyte counts.
Data analysis
The data analysis was performed by descriptive statistics indicators (frequency, percentage, mean, and standard deviation) and inferential statistical tests (Chi-square, ANOVA, and Repeated measurement) using SPSS software version 21. The significance level was considered as P <0.05.
Results and Discussion
In this study, 72 patients with COVID-19, divided into intervention (n = 37) and control (n = 35) groups were studied. There were 17 (48.6%) male subjects in the control and 19 (51.4%) male subjects in the intervention group. The results of statistical analysis showed that the intervention and control groups had no significant difference in terms of age, sex, BMI, smoking history, and occupation (P>0.05) (Table 1).
Time to symptom disappearance was assessed for the major symptoms. The time-to-event analysis revealed a statistically significant difference in 4 symptoms of cough, fever, dyspnea, and myalgia (Figure 1).

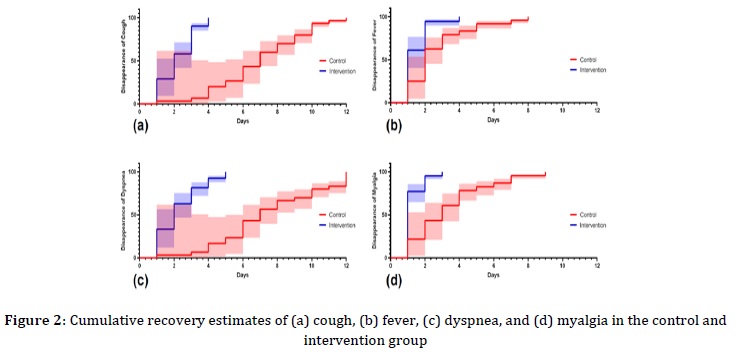
Where, median rate ratio of the cough disappearance in intervention group versus control group was 0.285 (CI95%:0.173- 0.427; P<0.05), rate ratio of the fever disappearance was 0.5 (CI95%:0.271 - 0.921; P<0.05), rate ratio of the dyspnea disappearance was 0.285 (CI95%:0.169 - 0.480; P<0.05), and it was 0.333 (CI95%:0.185 - 0.598; P<0.05) for myalgia disappearance (Table 2).
An evaluation of patients’ condition and daily vital signs were recorded and the averaged value of vital signs was compared between two groups. There was no significant difference in terms of averaged O2 Saturation, systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse rate (PR), and respiratory rate (RR) between the two groups (P>0.05), as presented in Table 3.
The trend of C-reactive protein in the group receiving herbal supplements decreased from the first to the fourth day, but then increased from the fourth to the fifth day, and thereafter decreased until the seventh day. However, compared with the intervention group, the control group had a significantly lower decrease in CRP in 7 days (P<0.05) (Figure 3).
The mean number of hospitalization days in patients in the herbal supplement group was significantly lower than the patients in the control group. Patients in the herbal supplement group were hospitalized for 4.12 days and patients in the control group were hospitalized for 8.37 days (Table 4). ICU admission and death only happened in 3 (8.6%) patients of the control group.


The results of this study showed that the addition of the proposed supplement (the combination of sugarcane, black myrobalan, and mastic) along with the treatment protocol of the Ministry of Health, can shorten the duration of treatment in patients with new coronavirus and relieve symptoms. While this study is the first study using this combination, no further studies with the same methodology and treatment method were available for comparison. Likewise, none of the herbs used in our combination was investigated in other studies as a treatment for COVID-19, except a clinical trial study in Iran, in which Anacyclus pyrethrum, Senna, Ferrula asafoetida, and Terminalia chebula effect have been planned to be tested on the COVID19 patients, but the study results are not yet reported. One of the main findings of this study was medication safety, as no subject showed any adverse events. This will help us to perform studies in a higher number of the patient or other groups of patients with more severe disease, underlying disease, and other age groups. The observed effects of the combination of sugarcane, black myrobalan, and mastic could be explained by the herb’s ingredients. In a 2008 study by Gupta et al. [23], a randomized double-blind clinical trial of 60 febrile patients using aspirin (60 mg/kg body weight per day) as a standard drug compared with the use of sugarcane plant was done. This trial showed that fever after oral administration of sugarcane at a dose of 60 mg decreased rapidly and significantly, and this effect on fever was more stable and significant than aspirin. This may be the reason for the sooner fever control in our intervention group. However, we were not able to monitor conventional antipyretic use in these patients and as a confounding factor, this was a limitation of our study; while for fever control, all patients had the same physician medical order.
The active ingredients of black myrobalan are terpenoids, carotenoids, flavonoids, alkaloids, tannins, and glycosides [24]. In studies, this plant has been mentioned as a rich flavonoid plant [25]. While we did not have the opportunity of chemical constituent compounds analysis of herbs, previous administrations of black myrobalan were shown to be safe in humans. AyuFlex herbal, manufactured by Natreon Inc., New Jersey, USA, is a US FDA approved black myrobalan supplement [26]. Black myrobalan has antiviral, antifungal, and antibacterial activity due to containing a variety of molecules. Molecules in this plant are gallic acid and 3-glycol glucose molecules that inhibit the process of HIV-1 integration [27-29], and thus prevent viral infection without any side effects. Black myrobalan extract is effective in inhibiting the division of cytomegalovirus and is useful in people with immune deficiencies [30-32]. The effect of using Black myrobalan has been shown against Respiratory syncytial virus, Hepatitis C virus, Herpes simplex virus, and Dengue virus [33]. It also eliminates salivary bacteria by inhibiting the glycolysis pathway [30]. Anti-inflammatory effects and improvement of asthma symptoms have been reported in the use of Terminalia chebula fruit extract. Animal studies showed that it can relieve cough even better than Codeine, a confirmed medication for the cough [31-33]. Furthermore, its anticaries properties may be as protection against tooth damage from daily sugarcane use, if the medication is going to be used for long periods [34]. However, this hypothesis needs to be evaluated in further studies.
Pistacia lentiscus resina (mastics), another component of our supplement, has antibacterial activity [35], prevents inflammation due to its Linalool content [36]. Studies show that mastics prevents the production of pro-inflammatory substances such as nitroxide and prostaglandin 2 (inhibition of cyclooxygenase 2 at mRNA and protein levels). Therefore, it is known as an anti-inflammatory and antioxidant substance [37, 38]. In laboratory studies on animal models, it has been shown that mastic plants can be effective in improving pulmonary fibrosis. The use of this plant extract in an animal model of asthma reduced airway inflammation by reducing the expression levels of TNF-α, IL-4, and IL-5, and improved pulmonary inflammation [39].
Study limitations
Our study had some limitations. First due to a low number of subjects, critically ill patients were not evaluated in this study. In addition, patients with the underlying disease were excluded. Further researches could be conducted on these populations as no significant severe effect was recorded. Another issue that should be taken into mind is that body response of different ethnicities widely might be different to virus itself or medications [40]. Furthermore, some people would require intensive care due to severe disease that we might not be able to easily apply herbal medicine [41].
Conclusion
The proposed combination of Sugarcane, Black Myrobalan, and Mastic seems to be effective in the symptom treatment and reducing the length of hospitalization in COVID-19 patients. Moreover, its safety was confirmed. Its application as a supplementary medication in COVID-19 treatment should be studied in more powered studies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
Abbreviations
Alanine aminotransferase (ALT), American Heart Association (AHA), Aspartate aminotransferase (AST), Blood urea nitrogen (BUN), Body mass index (BMI), Creatinine (Cr), Diastolic blood pressure (DBP), Hemoglobin (HB), International normalized ratio (INR), Partial thromboplastin time (PTT), Prothrombin time (PT), Pulse rate (PR), Red blood cells (RBCS), Respiratory rate (RR), Systolic blood pressure (SBP), Traditional Iranian Medicine (TIM), White blood cells (WBCS), Good manufacturing practices (GMP).
HOW TO CITE THIS ARTICLE
Samaneh Abiri, Alireza Hashemi Shiri, Mohammad Sadegh Sanie Jahromi, Esmaeil Rayatdoost, Hamid Afkhami, Ruhollah Ravanshad, Seyed Ehsan Hosseini, Navid Kalani, Parasta Heidari, Rahim Raoufi, Safety and Efficacy of Popular Iranian Herbal Cold Remedy for COVID-19: A Randomized Clinical Trial in Mild to Moderate COVID-19 Cases. J. Med. Chem. Sci., 2023, 6(8) 1799-1809


