Document Type : Original Article
Authors
1 Associate Professor, Department of Biotechnology, IMS Engineering College, Ghaziabad, UP., India
2 Ex-professor, Department of Biotechnology, IILM-CET, Greater Noida, UP., India
Abstract
Iodine deficiency leads to insufficient production of thyroid hormone which may cause many harmful consequences in humans. Owing to the cumulative impact of maternal, prenatal, and neonatal iodine deficiency, infants born with this condition can suffer mental retardation, developmental abnormalities, and disabilities. The present study is an effort to assess the functional role of β-sitosterol (phytosterol) and quercetin (flavonoid) in the brain development. An iodine-deficient rat model was developed by feeding 0.025% of methimazole (MMI). These hypothyroid-induced young adult female Wistar rats were followed by mating with healthy male rats. During the whole gestation, these pregnant hypothyroid-induced females received treatment with β-sitosterol (150 mg/kg/day) and quercetin (150 mg/kg/day), both separately and together. Their little born was observed based on hematological profile and behavioral study. RBC, hemoglobin, and total protein level in pups born to a hypothyroid-induced mother were found lower than in the control group. However, it has been observed that the combination of β-sitosterol and quercetin normalises the level of RBC, hemoglobin, and total protein. Motor coordination and memory functions in this iodin-deficient model were found below the normal range. It has been observed that in the iodine-deficient brain, β-sitosterol and quercetin had a significant effect on motor coordination and spatial memory. The brain development of pups born to a hypothyroid-induced mother revealed encouraging effects with the synergistic action of quercetin and β-sitosterol.
Graphical Abstract
Keywords
Main Subjects
Introduction
Congenital hypothyroidism, previously known as Cretinism, develops in newborns whose mothers were iodine deficient during pregnancy. A good number of research works has been carried out to evaluate the effect of moderate iodine shortage during pregnancy. Although iodine deficiency in pregnancy affects both the mother and child, the consequences of iodine deficiency in pregnancy and its effect on child health have received little attention.
Iodine is required for the generation of thyroid hormones such as thyroxine (T4) and 3,5,3-triiodothyronine (T3), which are important for appropriate brain and central nervous system development [1]. The effects of thyroid hormones on the brain development and function are an important area of interest to the researchers. Apart from brain development, these hormones influence different aspects of neuronal cell proliferation and differentiation, signaling, and glial cell differentiation [2].
The function of thyroid hormones in the brain development has been widely examined in hypothyroid mouse models. Hypothyroidism can be induced by thyroidectomy, radioactive iodine, or the administration of synthesis inhibitors like propylthiouracil (PTU) and methimazole (MMI) during pregnancy and lactation. Throughout the key stages of brain development, i.e. during prenatal and neonatal life, thyroid deficiency diminishes cell quantity, synaptogenesis, and dendritic arborization. Thyroid deficiency further affects cell migratory pattern and inhibits axonal myelination [3]. The primary thyroid hormones, T4, and T3 influence brain growth and maturation via connecting to the T3 nuclear receptor from the early inception of pregnancy [4, 5].
The recent studies have shown that plant based products like phytosterols, flavonoids, flavanones, etc. have been proved to be very useful in the treatment of developmental disorders of brain caused by hypothyroidism. β-sitosterols are eminent group of phytosterols present in several lipid rich plants such as olive, nuts, etc. They have a structure similar to cholesterol and have been reported to be of great biomedical applications especially in therapeutics [6]. Reports suggest that β-sitosterols have multiple vital therapeutic roles such as anti-inflammatory, anti-microbial, anti-cancerous, anxiolytic, and sedative effects [7, 8]. As cholesterol is an essential component for the regular functioning of the neurological system, both during development and in the adult life [9], it is actively generated in the CNS of humans and the other animals during the first few weeks after birth, and any disruption in its production and supply during this newborn period might contribute to neurodegenerative diseases. Cholesterol is essential for biological processes such as microtubule stability, synaptogenesis, neurite outgrowth, myelination, and glial cell proliferation [10]. Hence β- sitosterols a plant based product with the similar efficiency as cholesterol and very low hazardous effect on health makes it a potential compound for the related studies.
Flavonoids, another important plant based compound, have been studied by different research groups for its varied biomedical applications. Quercetin is a ubiquitous flavonoid found in various vegetables and fruits [11, 12] which has various functions, including anti-inflammatory, neurodegenerative protection, and anti-cancer effects [13, 14]. In many in vivo experiments, quercetin has been demonstrated to reduce neuronal apoptosis generated by various cytotoxic treatments, including a fatal oxidative stress stimulus [15, 16]. Quercetin also inhibits neuro-inflammation and is effective in the treatment of neurological diseases [17].
sitosterols and Quercetin are two important phytosterol and flavonoids, respectively, shown various beneficial functions in human body. It has been observer that if the mother has sever hypothyroidism, it can cause problems in brain development in the baby, leading poor motor coordination, memory impairment, lower IQ, and language skills, etc. As both the compounds show neuro-degenerative protection, it has been thought to see whether these compounds alone or in combination can improve the disorders of developing brains caused by hypothyroidism or not. Rat is a well-established model to understand the effect of fetal and postnatal thyroid hormones on brain development [18-20].
Materials and Methods
All the chemicals used in this study were purchased directly from Sigma Aldrich. The ethical approval for the study was obtained from IAEC – (IIRT/IAE/2019/036).
Animal model
In the current study, Wistar female young adults were used as subjects. Rats were housed in humane conditions with 12-hour sun and 12-hour dark cycles at 22-25n °C after receiving permission from our Institute's ethical committee and following Veterinary Management guidelines.
Experiment design
Development of iodine deficit rat model
For the study, 50 days young female rats (weighed 120–150 g) were selected. They were treated with thyroid peroxidase inhibitor methimazole (MMI) at a concentration of 0.025% in drinking water. The treatment was continued for twenty-one days. This caused hypothyroidism in the rats. After twenty-one days of intervention, blood (about 0.5 mL) was removed from the retro-orbital complex while being lightly sedated with ether. At -20 °C, the plasma was spun out and maintained. Body weight recorded every 5 days throughout the experimental periods.
Principle: Total triiodothyronine (TT3) and total thyroxine (TT4) in serum were measured using a solid-phase radioimmunoassay through a Coat-A-Count Package (DPC, Los Angeles, CA), in which T4 or T3 and 125I-labeled T4 or T3 competed for the antibody binding sites in the sample for a set amount of time. The availability of 8-aniline-1-naphthalene-sulphonic acid (ANS) is necessary for the release of connected T3 from protein carriers, causes this reaction to occur. As both free and protein-bound TT4 and TT3 from the study sample are able to compete for the antibody binding sites with radio labeled TT4 or TT3, the assay tests TT4 or TT3.
Procedure
From female rats, blood was taken. After an hour at RT, the serum was extracted from the blood using centrifugation. The bottom of the antibody-coated tube received the pipetted serum sample. Each tube received 1.0 cc of 125I-labeled TT4 or TT3. The reaction mixture was vortexed, and it was then kept at 37 °C for an hour. After properly emptying the tubes of the mixture using a decanter, they were counted for one minute at the counter. The standard curve was used to compute the values of the unidentified samples.
Animal Grouping and Treatments
The normal adult male rats of the same strain were mated overnight with the iodine-deficient female rats (one male: three females). Females who tested positive for sperm were randomly assigned to four test groups. The normal female rat was further mated with the male rat of the same strain for the control group. There are five study groups and each group have three pregnant rats.
β-Sitosterol and quercetin were initially diluted in DMSO (dimethyl sulfoxide) to create the stock solution due to their limited solubility in water. From this stock, a fresh aqueous solution of β -Sitosterol and quercetin was made every day in the quantity needed for an oral dose. Rats in Groups 1 (Control groups) and 2 (hypothyroid group) that were pregnant received 0.1% DMSO treatment. Rats in Group 3 that were pregnant received treatment with quercetin at a dose of 150 mg/kg. Rats in Group 4 that were pregnant received treatment with 150 mg/kg of -sitosterol. Group 5 pregnant rats received treatment with 150 mg/kg each of -sitosterol and quercetin. From the first day of pregnancy through the twentieth day of pregnancy, all groups received a single daily gavage treatment (GD1-GD20). Every pregnant rat was permitted to give birth and raise her young ones.
On postnatal day 16 for hematological investigation and on postnatal day 40 for behavioral testing, the pubs (n=5) were randomly selected from each group.
Hematology analysis of pubs
The blood was pooled from litters of each intervention group (n=5) at postnatal day 16 through the cardiac puncture in anesthesia. The complete blood profiles with biochemical parameters were analyzed.
Behavioural study
To investigate the effect of quercetin and β-sitosterol on motor coordination and memory functions, we performed a behavioral study (n = 5/group) using a Rotarod performance and Y-maze task.
Motor coordination of pubs was tested
Rotarod performance, as a measure of motor activity, was measured in rats on 40th days. In each series, the rats were trained on an accelerating (at constant speed, 12 rpm) rotating rod (rotarod IITC, life science Rota Rod series 8) for 3 days. The total score was calculated as the mean period (in seconds) over three trials that a rat was able to stay on the rod.
Spatial memory test in different groups
To test spatial memory performances, we performed a Y-maze test at P40. This test is focused on rodents' innate drive to discover new worlds and to test the memory of short-term spatial awareness. Training happens with three white, opaque plastic weapons at a 120° angle from each other in a Y-shaped labyrinth. The animal is enabled to freely navigate the three arms since its entrance to the middle of the labyrinth. The topic should demonstrate a propensity to join a less recently visited arm throughout several arm inputs. During Y-maze test if rat trace the light arm (i.e. non correct arm) within 30 sec the attempt was noted as a positive response. If rat not trace the light arm within 30 sec the attempt was noted as a negative response.
Statistical analysis
At least three duplicates of each experiment were run, and the resulting data were gathered. Data analyses were carried out using Windows Microsoft Excel, and the results were given as Mean ± S.D. (Standard Deviation). The comparisons between the two groups were made using independent sample t tests. P-value of <0.05 was regarded as statistically significant.
Result and Discussion
Insufficient availability of iodine, during brain development, leads to a significant reduction in the mental growth of the individual. Iodine is a required micronutrient for thyroid hormone production and is required for human and animal bodies. Thyroid hormones, Triiodothyronine (T3), and Tetra- iodothyronine (Thyroxin or T4) are iodinated amino acids produced and secreted by the thyroid gland with a major effect on physiological (growth metabolism and thermoregulation) and developmental processes [21]. T4 is the necessary substrate for the ontogenically controlled generation of T3 in the quantities required for the optimal growth, both temporally and spatially, in different brain structures. Figure 1A and 1B represent the TT3 and TT4 levels of euthyroid and hypothyroid groups, respectively. It has been observed that in the MMI treated group the level of TT3 and TT4 is significantly lower than the euthyroid group (control). The results confirmed the hypothyroidism induction in treated rats, as compared with the control group. No significant change was observed in body weight in MMI treated group when compared with Euthyroid rats' body weight. However, the significant changes in hair texture and density were observed under hypothyroidism. The scores of animal studies have provided insight into the mechanisms of TH-dependent brain development. Studies on hypothyroid neonatal rats have shown that a lack of TH in the sensory cortex, prefrontal cortex, cerebellum, auditory cortex, and hippocampus induces reduced axonal development and dendritic arborization [22, 23]. According to certain experimental investigations, maternal thyroid hormone shortage during pregnancy may result in the irreparable brain damage in the fetus, leading to the varied degrees of mental retardation, cognitive impairment, and learning and memory issues [24, 25].

In this study, the potential effects of phytosterols and flavonoids on the developing brain of pubs born to hypothyroid-induced mother rats were observed.
The association of thyroid hormone disorders and abnormalities in hematological parameters is well-known. Horton et al. [26] observed a decreased number of red blood cells (RBCs) in the peripheral blood (PB) of hypothyroid patients. Nabavizadeh et al. [27] also observed the changes in red blood cells (RBCs) and hemoglobin (Hb) content of hypothyroid induced rats. The estimated hematological parameters in the hypothyroid pups, such as RBC, Hb, PCV%, and total protein content, differed considerably from those produced during therapy and in the control pups. The complete blood profiles and biochemical parameters are various hematological parameters were calculated in control and all treated animals. Table 1 presents complete blood profile of the all group pubs. The projection low levels of the hypothyroid condition were shown to have an impact on the RBC and hemoglobin, but a combined dose of quercetin and -sitosterol seems to normalise the level of RBC and Hb and has proven to be quite helpful. When Quercetin and β-Sitosterol were coupled in the treatment, the PCV% levels were discovered to be closely related to the control values. WBC, reticulocyte, clotting time, platelet count, and leukocyte count were all at similar levels in the phytosterol and flavonoid-treated group as well as control group.
Table 2 lists the biochemical parameters of the all group pubs. Total protein level was found significantly lower in hypothyroid groups and appeared to return to normal with Quercetin and β-Sitosterol treatment group than the individual treated group. Other biochemical parameters, including glucose, albumins, SGOT, SGPT, BUN, all salts, and choline esterase were found to be least influenced by hypothyroidism. Nonetheless, Quercetin and β-Sitosterol treatment brings it closer to the control group.

Various studies have been conducted on the effect of phytosterols and flavonoids in various brain disorders. Dash et al. [28] found that phytosterols had adequate capacity to reduce inflammation and neurodegenerative disorders. One research group has demonstrated that β-sitosterol enhance vanadium induced neurotoxicity by improving motor coordination through antioxidant activity [29]. Figure 2 displays the Rotarod performance of all group pubs at postnatal day 40. Test result indicated that there was a significant loss of motor co-ordination under hypothyroidism condition, as compared with the control group. The time of stay on rotating rod has decreased significantly (#P<0.05), while a significant improvement (*P<0.05) was seen in quercetin + β-sitosterol treated groups, as compared with hypothyroid group. This observation is similar to that of control group. However, the improvements in performance were further observed in quercetin and β-sitosterol treated group, as compared with hypothyroid rats. The results of the present study show that combined treatment of quercetin + β-sitosterol alleviates the hypothyroid-induced impairment of motor coordination.

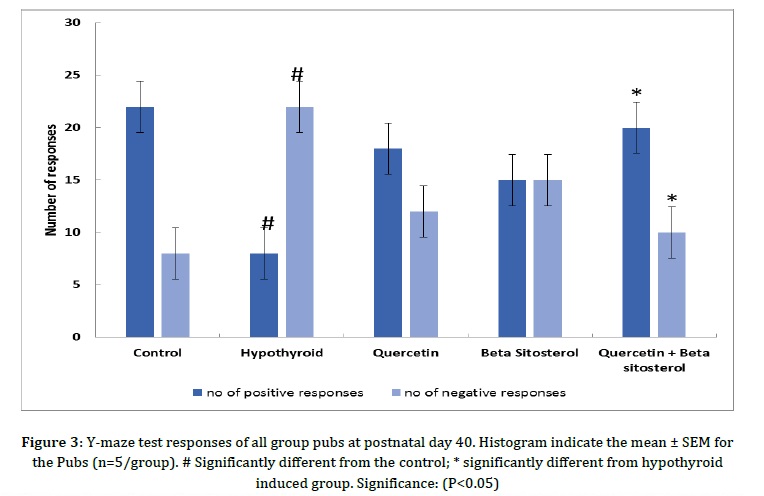
Wang et al. [30] proposed that membrane cholesterol/β-sitosterol replacement facilitates nonamyloidogenic APP (Amyloid Precursor Protein) production and is related to the re-distribution of APP amongst lipid rafts and non-raft areas. It is worth to investigate the medicinal potential of β-sitosterol supplementation for neurodegenerative condition prevention and treatment. Similar to our work, Wang Q et al. [31] conducted a study on the effect of quercetin and indicated that quercetin potently alleviates maternal LPS exposure induced fetal brain injury in rats, making it a potential therapeutic for suppressing infant brain damage as a result of maternal infection. According to Jian et al. [32], β-sitosterol improves memory and learning impairment in APP/PS1 mice. Figure 3 represents Y-maze test responses of all group pubs at postnatal day 40. The Y-maze test findings indicated that there was a significant loss of memory i.e. the significant decrease in the number of positive responses (#P< 0.05) and a significant increase (#P<0.05) in the number of negative responses under hypothyroidism condition with respect to control group, while a significant improvement (*P< 0.05) was observed inquercetin + β-sitosterol treated groups, as compared with hypothyroid group. Improvements in performance were also observed in quercetin, and β-sitosterol treated group, as compared with hypothyroid group rats. The maximum improvement was seen in synergistic group.
It was observed that hypothyroid-induced memory dysfunction was reversed by the synergistic effect of quercetin+β-sitosterol, indicating that a combination of phytosterol and flavonoid would be beneficial to improve motor coordination and memory functions. This animal study proved the efficacy of phytosterols and flavonoids to be used in brain development, but further human trials need to be conducted to establish its full potential for human use. We, therefore, recommend that potential in depth study is required to see the effects of phytosterols and flavonoids on brain-related disorders.
Conclusion
The main focus of this study is to understand the synergistic potential of phytosterol and flavonoids on brain development under hypothyroidism. In all types of intervention, the behavioural pattern like motor coordination, memory function, and hematological parameters were examined. The results are in line with our proposed hypothesis that the synergistic effect of quercetin and β-sitosterol prevented hypothyroid-induced developmental disorders of the brain, such as loss in motor coordination and memory impairment, in pubs born to hypothyroid-induced mothers. Combination of phytosterol and flavonoid also prevent the losses of total protein, RBC, and hemoglobin content due to the iodine deficiency. These results suggest that plant based products with the significant potential bioactivity would be more beneficial against neurological disorders caused by nutritional deficiencies. However, more advanced animal and human studies are required to validate the preciseness of this result.
Acknowledgements
The authors thank Dr. Amit Pal and Dr. Pawan Kumar from Institute for Industrial Research and Toxicology (IIRT), Ghaziabad for Institutional Support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
HOW TO CITE THIS ARTICLE
Rashmi Chandra, Chaiti Ganguly. Effect of Phytosterols on Brain Development in Iodine Deficient Rat Model- A Comparative Study. J. Med. Chem. Sci., 2023, 6(7) 1696-1705


