Document Type : Original Article
Authors
1 Department of Basic Science, College of Dentistry, University of Baghdad, Baghdad, Iraq
2 Medical Microbiology, Department of Basic science, College of Dentistry, University of Baghdad, Baghdad, Iraq
Abstract
Honey is one of the most products has been used as an antimicrobial agent since the ancient times, nicotine is an important cause of dental caries and oral diseases, the purpose of this research is to study the effectiveness of honey and nicotine on the growth, acid production and biofilm formation of salivary mutans streptococci (M.S.).
Materials and methods: Saliva samples from dentistry students were used to isolate M.S. In this investigation (age range between 21-23 years old). The morphological characteristics and biochemical tests were used to isolate, purify, and identify these microorganisms. The diameter of inhibition zone of honey and nicotine was determined by agar diffusion method and the pH measured by pH meter.
Results: Results from an agar diffusion experiment showed that both honey and nicotine inhibited the growth of Mutans streptococci, with the diameter of the inhibition zone growing larger with increasing concentrations of both substances. However, the nicotine effect was significantly weaker than that of honey. Honey had a bactericidal concentration of 90%, whereas nicotine's was 45 mg/ml. Likewise, the honey and nicotine was high significant inhibit (P<0.01) the growth of M.S in vitro. Furthermore, the nicotine have a negative effect on honey activity on inhibition the acid product.
Conclusion: In our study, we concluded that honey have indicated strong effects in inhibit M.S acid production. On the other hand, nicotine increased acidity and metabolic activity of M.S in the presence of honey. Therefore, smokers with a high consumption of nicotine should consider to the honey to minimize their chance of developing dental caries by reducing acid production from M.S streptococci.
Graphical Abstract
Keywords
Introduction
Caries in the teeth are caused by a combination of variables but not limited to dental plaque (microorganisms), carbohydrates (substrates), a vulnerable host (teeth), and time [1]. The oral flora often include this gram-positive and facultative anaerobic bacteria. Streptococcus mutans has been linked in several studies to the onset of a carious lesion, and isolation of this bacterium from dental plaque near a carious tooth surface has been shown to be possible [2]. The microorganisms that have adapted to life on teeth produce an acid by fermenting carbohydrates, which demineralizes the mineral portion of teeth hard tissues and eventually breaks down the organic matrix holding the bacteria together. This film, called dental plaque, can be seen on the tooth surface [3]. The concept that plants may be used for medical purposes is quite old. Since prehistoric times, people on every continent have used poultices infused with hundreds, if not thousands, of local plants [4]. Antimicrobial agents have been recommended for years as a means of preventing plaque and oral illness. Oral rinsing and tooth-brushing are only two examples of the topical applications used to provide various chemicals. Some chemical compounds have shown promise in reducing plaque buildup and, by extension, in reducing caries [5]. There is a slimy coating of biofilm on the tooth surface made up of millions of bacterial cells, salivary polymers, and food particles. If left unchecked, this biofilm may spread to cover hundreds of tooth surfaces. Many different types of bacteria may colonize and thrive on the biofilm (also known as plaque) that forms as a result of this process [6]. Tooth decay, or dental caries, is a chronic and progressive condition characterized by the gradual loss of tooth hard tissue due to acid breakdown. About 96% of tooth hard tissue is hydroxyapatite, which becomes soluble at pH levels below 5.5 [7]. Despite the fact that many antibacterial medications have been touted as effective in preventing caries, they work by killing off most of the bacteria in the mouth. This destroys the normal, or health-related oral flora, and can result in several defects in the oral ecosystem, such as the development of resistant strains and opportunistic infections [8]. Gram-positive ovoid cocci that normally form pairs or chains, are aciduric (they thrive in acid medium) and acidogenic (they make acid) and are non-motile facultative microorganisms are known as S. mutans [9].
The ideal growth temperature for anaerobes is 37 C. Thus, it would be more efficient to target bacterial cariogenic factors as an antibacterial method for preventing caries without endangering the existence of indigenous bacterial species in the oral environment [10]. The World Health Organization (WHO) has called for further research into the potential novel antibacterial chemicals and natural bioactive substances. These infections are notoriously difficult to treat with traditional antimicrobials due to issues including medication resistance and unwanted side effects. Treatment of multi-drug-resistant (MDR) bacteria sometimes requires the use of synergistic antibiotic medication combinations with bioactive substances [11]. Honey is a complex mixture containing many elemental components, which depends on the geographical and botanical origin [12]. Honeybees obtain most of the necessary carbohydrates from nectar, whereas the proteins, fats, minerals, and vitamins [13]. Honey possesses inherent antimicrobial properties, which are high osmotic pressure/low water activity, in which the low water activity of honey is inhibitory to the growth of the majority of bacteria, and to many yeasts and moulds. When applied topically to wounds, osmosis would be expected to draw water from the wound into the honey, helping to dry the infected tissue, and reduce bacterial growth [14]. Nicotine is an alkaloid and is present in the leaves of tobacco [15]. Nicotine is not a carcinogen, but a compound that have greatest influence on a smoker’s cancer risk. Nicotine sustains tobacco addiction and continued smoking [16]. The effects for oral health are related to both teeth and gums. The main cause of dental caries is the bacteria Streptococcus mutans [17].
The aim of the study was to determine the anti-microbial activity of honey and nicotine extracts on acidity of salivary mutans streptococci.
Materials and Methods
Forty samples of bacteria were obtained from stimulated saliva samples that were taken under standard settings. According to Tenovuo [18], dental students without a significant medical history between the ages of 21 and 23 years old were recruited for this investigation. Sterile normal saline was used to create ten-fold serial dilutions. Mitis-salivarius bacitracin agar (MSB Agar) was inoculated with two different dilutions, and then the jar was placed in an anaerobic incubator with a gas pak for 48 hours at 37 °C, followed by another 24 hours of aerobic incubation at the same temperature. Each inoculum was prepared by transferring a single colony of MS bacteria into 10 ml of sterile BHI-B and incubating the mixture aerobically at 37 °C for 24 hours.
Preparation of honey and nicotine for experiments
The honey is taken from farms in Iraq, Baghdad, Al-Madden, in the month of February and it is prepared in the form of different concentrations by serial diluting in distilled water and sterilize by Millipore filter (0.40 µm), and then by (0.20 µm). Nicotine was purchased from the American market, ready to use, with a concentration 200 ml prepared by serial dilution with distilled water and sterilizer by Millipore filter (0.20 µm).
Sensitivity of mutans streptococci to different concentrations of honey and nicotine by well-diffusion method
To investigate the sensitivity of MS to honey and nicotine; the final concentrations 70, 80, 90, and 100% of honey were prepared in distal water. The different concentrations 15, 25, 35, and 45 mg/mL of nicotine were also prepared in distal water; well-diffusion technique was used in this experiment to detect the diameter inhibition zone on nine MS [19].
Determination of the Minimum Bactericidal Concentration (MBC) of honey and nicotine
In this experiment, the brain heart infusion broth and serial dilution of both honey and nicotine were used. The MBC was determined as the lowest concentration of agents that kill mutans strptococci cells [20].
Determine acid production by M.S. isolates with home and nicotine
In this experiment, the ∆pH was measured to study the effects of honey and nicotine on the acid production of mutans streptococci [21].
Results and Discussion
There was not a single gram-negative isolation (Figure 1). Direct smear microscopy without staining revealed that none of the isolates were motile and tests for the presence of the enzyme catalase showed that the isolates were also catalase negative. MS's fermentation of mannitol was tested in cysteine trypticase-mannitol medium.
Determination antimicrobial activity of Honey
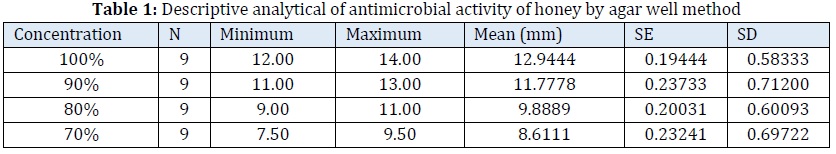
In this study, the results showed the nine isolates of Mutans streptococci were sensitive to the honey and nicotine respectively and the diameter of the inhibition zone was increased when the concentrations of the extracts increased. Tables 1 and 2 show the minimum, maximum, and mean of inhibition zone.
At the same time, in a comparison between the activity of honey and nicotine extract by ANOVA test for each concentration in relation to M.S shows a highly significant difference in which (F-value), (P-value) between other concentrations as indicated in Tables 3 and 4.

Minimum bactericidal activity of honey and nicotine against M.S
The minimum bactericidal concentration (MBC) of honey and nicotine for M.S were determined, as presented in Table 5. The MBC range for honey and nicotine was 80-90% and 25-45 mg/mL, respectively
Analysis of the effects of honey and nicotine, on pH by ∆pH
In this study, the impact of honey and nicotine, in 1/2 MBC on acid generation was evaluated by pH before and after incubation with M.S, as diplayed in Figures 1 and 2. The results of this study showed that the addition of agents (honey and nicotine,) decreased the mean ∆pH values compared with the bacterial broth without these extracts. The mean, maximum, minimum, standard deviation, and standard error values of the honey and nicotine compared with control were also presented in Table 6.
The normality distribution test (Kolmogorov-Smirnova and Shapiro-Wilk) was used to compare the effects of compounds (honey and nicotine,) on nine M.S isolates regarding the ∆ pH decreases. The results showed that all tested agents (honey and nicotine) reduced ∆ pH, but there was no significant difference ∆ pH decreases as shown in Table 7.
Regarding the ability of plant extracts to ∆ pH decreases, the tested extracts display different degrees of ∆ pH decreasing activity, when comparison of the activity of tow compounds (honey and nicotine) in reducing ∆ pH reveals a highly significant difference between them in which (F-value =155.285) and (P-value 0.001), as listed in Table 8.



Antibacterial Effects of the Plant Extracts
The most of tested plant extracts showed antibacterial effect, which may indicate the antimicrobial effect of plant biological activity substances that restrict bacterial growth. In this study, honey and nicotine extracts were showed activity against M.S.
Concentration-dependent inhibitory effects of honey and nicotine on M.S
Inhibitory effects of the tested extracts on Mutans streptococcus growth were measured via serial dilution. Honey is known to have potential of antibacterial activity. The results of this study found the defected M.S growth and inhibition zone increase when the honey concentration increased [22] and ANOVA analysis for each concentration in relation to the M.S growth shows highly significant difference (P- value <0.001). Although honey's antibacterial activity has been studied extensively, its precise method of action is still unclear. This is because honey may have a few different assault sites, each of which may be effective depending on the specific bacterium. The osmotic pressure is increased, which may slow bacterial growth in response to the high concentration of sugars (80%) [23]. Our results were in agreement with the previous investigation studies. For example, this study noticed, the Gram-positive bacteria are sensitive to secreted by the honeybee and one of the most important enzymes involved in honey ripening processes is glucose-oxidase, which can transform glucose to gluconic acid and the side product of this reaction, hydrogen peroxide is a strong antibacterial agent [24]. The other study indicated the honey have antimicrobial agent like H2O2 that inhibit growth of bacteria [25]. Micro dilution method was used to determine the lowest plant extracts concentration that killing the bacteria cells and found effective in the MBC evaluation. At a concentration of 90%, the MBC value of honey extract was found to be effective against mutans streptococci and all isolates were killed at this concentration. It appears to be harmful to these bacteria, as indicated by a reduction in their growth on the brain heart agar plates. The effect different concentration of nicotine on the growth of M.S isolates, in the current study findings, the nicotine extract had an antibacterial effect on all nine isolates at high concentrations (15–45 mg/mL). The concentration (45 mg/mL) of the nicotine has an antibacterial effect against M.S with a mean diameter of inhibition zone (8.6111 mm) compared with the other concentrations (15 mg, 25 mg, and 35 mg/mL) and highly statistically significant between different concentration (p-value 0.0001).
The nicotine in a low concentration increased the M.S adhesion and growth, but in high concentration it kill the M.S [26]. The nicotine extract was active exhibiting the high potency with MBC from 45 mg/mL inhibit the growth of 9 isolates from mutans streptococci and this confirms of the need for a high concentration of nicotine to be antitoxic effect of the bacteria. Our results were in disagreement with several studies. For example, these studies noticed the MBC of nicotine is 32 mg/mL effect on the gram-positive bacterial cells [27]. This may be depending on the type of nicotine used, the manufacturer of nicotine, and the type of M.S isolate.
The impact of honey, and nicotine extracts on the acidogenicity of Mutans streptococci
Results of this study indicated the mean, the maximum, the minimum, standard deviation, and standard error values of honey. Nicotine, that the ∆pH mean values decreased with the addition of plant extracts (honey and nicotine) compared with the bacterial broth without these extracts.
The ability of honey and nicotine extracts to either prevent or lessen M.S. acidogenicity improved with increasing concentration. A possible mechanism for this action is the suppression of carbohydrate-fermenting and acid-producing enzymes. S. mutans may create acids for metabolizing carbohydrates and these acids lower the pH of the biofilm, leading to demineralize tooth structure [28]. Honey are other sources of flavonoids, a class of pigments made by plants. Flavonoids were shown to greatly reduce the activity of MS F-ATPase [29].
In response to low pH, S. mutans has developed mechanisms to minimize the impacts of acidification by increasing proton-translocating F-ATPase activity [29]. F-ATPase transfers protons (H+) out of cells in connection with ATP hydrolysis to keep intracellular pH higher than external Ph [30].
Proton translocating F-ATPases (H1-ATPase) have important functions in preventing M.S from environmental stress produced by acidification. F-ATPases assist control ΔpH across the membrane by taking in or releasing H+ as they manufacture or hydrolyze ATP, which is necessary for glycolysis function and because the sugar transport system is susceptible to the cytoplasmic acidification [31].
Was found that the addition of nicotine extract decreases the effectiveness honey extracts for reducing ∆ pH. This study suggested that increasing the concentration of nicotine extract increased the metabolic activity of M.S, increasing the majority of glycolytic pathway metabolites, resulting in significantly elevated lactate, causing an increase in ∆ pH and possibly contributing to caries formation. Likewise, the presence of nicotine may be block the anti-acidity of honey.
Conclusion
- Honey and nicotine at different concentrations have the antimicrobial activity against salivary mutans streptococci and their activity increased with the concentration increased. The MBC was 90%, 45 mg/ml, respectively.
- Honey and nicotine have anti-acid production from mutans streptococci.
- Nicotine increased acidity of mutans streptococci in the honey presence. To decrease their risk of dental caries caused by mutans streptococci, smokers who consume a lot of honey may choose to switch to the alternative soft drinks and sweeteners.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
ORCID:
Abbas N. Al-Shamary
https://orcid.org/0000-0002-5081-4200
Abbas S. Al-Mizraqchi
https://orcid.org/0000-0001-9787-5708
HOW TO CITE THIS ARTICLE
Abbas N. Al-Shamary, Abbas S. Al-Mizraqchi. The Combination Effects of Honey and Nicotine on the Acid Production of Oral Mutans Streptococci. J. Med. Chem. Sci., 2023, 6(6) 1410-1418


