Document Type : Original Article
Authors
Department of Pharmacognosy, SRM College of Pharmacy, SRM University, Kattankulathur, Chennai, Tamil Nadu, India, 603203
Abstract
Rourea minor is a medicinal plant with anti-diabetic properties, and it can be found in South and North Asian countries. The study design involves the preparation of an ethanol Rourea minor stem extract; investigation of its oral glucose tolerance test, body weight, blood glucose, hematological, lipid profile, liver and kidney parameters, enzymatic oxidative parameters, and histopathology of the liver, kidney, and pancreas. Streptozotocin (55 mg/kg b.w) was given intraperitoneally in a single dose and was used to cause diabetes in male Wistar rats. Diabetic rats were treated with 200 and 400 mg/kg body weight of Rourea minor stems ethanol extract, and standard Glibenclamide (5 mg/kg b.w/day) for a period of 21 days. Oral administration of Rourea minor stems ethanol extract did not exhibit toxicity and death at a dose of 2000 mg/kg b.w. Rourea minor stems ethanol extract treated diabetic rats significantly (P<0.0001) reduced elevated blood glucose, glycolated heamoglobin, creatinine, urea, low-density lipoproteins, very low-density lipoproteins, alkaline phosphatase, serum glutamic pyruvic transaminase, serum glutamic-oxaloacetic transaminase, glutathione, glutathione reductase, glutathione S-transferase, and malondialdehyde levels when compared with diabetic control rats. The body weight, protein, albumin, hemoglobin, red blood cells, white blood cells, and high-density lipoprotein levels were significantly (P<0.0001) increased in diabetic rats treated with Rourea minor stems ethanol extract, as compared with diabetic control rats. The present study results confirm that Rourea minor stems ethanol extract possesses significant anti-diabetic and anti-oxidant activities in diabetic conditions.
Graphical Abstract
Keywords
Main Subjects
Introduction
Persistent hyperglycemia is induced by low insulin production, insulin action, or both, thereby leading to impaired carbohydrate, lipid, and protein metabolism [1]. According to the recent assessment of the prevalence of diabetes worldwide, 463 million individuals are currently suffering from diabetes. This would affect 9.3 percent of the global population (463 million people) in 2019, rising to 10.2 percent (578 million) by 2030 and 10.9 percent (700 million) by 2045, according to the report [2]. As most of the currently available drugs have a variety of side effects, the effective treatment of Diabetes Milletus (DM) is regarded as a critical task for the medical community [3]. Several studies have investigated non-traditional therapeutic drugs with similar therapeutic efficacy but with fewer adverse effects than typical therapeutic agents. Various researchers have attempted to find alternatives to traditional therapeutic agents with similar therapeutic efficacy to the conventional therapeutic agents, but do not have as many side effects [4]. However, no alternative treatment options for DM have been recommended. Ethnobotanical knowledge contains 1200 medicinal plant species that may be used to treat diabetes and its consequences [5]. Natural products that lower metabolic syndrome and diabetes-related morbidities, such as oxidative stress and chronic systemic inflammation, help patients. Natural diabetic remedies, on the other hand, could be a valuable addition to existing therapy. Controlling hyperglycemia in diabetic patients is essential for lowering the risk of micro-and macrovascular complications [6]. Various resources from nature play a vital role in diabetes mellitus therapy, particularly in underdeveloped countries, by delaying the onset of diabetic complications and correcting metabolic abnormalities [7]. Rourea minor Gaertn., a climbing shrub tree of the Connaraeae family, is widely spread in tropical Africa and is used to treat various disorders [8]. R. minor can be found throughout Southeast Asia. Rheumatoid arthritis patients with rheumatoid arthritis after childbirth have traditionally been treated with R. minor young stems found in North Asian countries. The roots and twigs are used to make a bitter tonic and a uterine tonic [9, 10], from the stems of R. minor, rourinoside, and rouremin are isolated. R. minor stems have been used to separate Bergenin and catechin [11]. R. minor stems extract has been synthesized into silver nanoparticles [12]. The root portions, R. minor has been found to have anti-diabetic activity [13] and a hypoglycemic effect [14]. Only two researches on R. minor stems were found in the literature review. We intend to examine the anti-diabetic and antioxidant effects of ethanol extract of young stems of Rourea minor in diabetes conditions based on the traditional usage chosen for these Rourea minor (twigs).
Materials and Methods
Collection of plant material and authentication of Rourea minor
The stems of the healthy plant, Rourea minor, were collected from Kerala. PARC/2020/4367 is the authenticated voucher number. The remaining pieces of the stem were sun-dried, ground into a coarse powder, and used for further research.
Preparation of the extract
The R. minor stem powder drug (60 g) was obtained from various organic solvents in a sequence of increasing polarity, like ether of petroleum, ethanol, and ethyl acetate. The extraction procedure was carried out three times. The rotary evaporator was used to evaporate most of the solvent under reduced pressure. A dark greenish-brown semisolid extract weighing 10 g was obtained.
Chemicals
Streptozotocin was obtained from Lab Chemicals, Chennai, India. Glibenclamide was procured from Sigma-Aldrich, Hyderabad. The determination of all the parameters was performed in Bangalore laboratories.
Animals
The trials were conducted on albino male Wistar rats, weighing 170-200 g, were used. Animals were kept in the SRM College of Pharmacy's Animal House under controlled environmental conditions (23±2 °C), humidity (45-55%), and a 12-hour dark cycle, with consent from the Institutional Animal Ethical Council (Reg.no.IAEC/257/2021). The animals were given a pellet diet to eat (mass biotech, Chennai).
Acute toxicity
An acute toxicity investigation was carried out by OECD 423 recommendations. Male albino Wistar rats were used in this experiment. Test medications were given orally to overnight starved rats at a dose of 2000 milligrams per kilogram of body weight. The animals were subsequently monitored continuously during the following four hours and then every 24 hours for general behavioral, neurologic, and autonomic characteristics, and mortality. A dosage of up to 2000 mg/kg of body weight is permissible, the extract was found to be safe.
Induction of diabetes
STZ (55 mg/kg b.w) was dissolved in distilled water and injected intraperitoneally (i.p) once. It was used to investigate induced diabetes mellitus in overnight-fasted rats [15]. Fasting blood sugar levels have been measured over the past 3 days. All of the rats had their blood sugar levels measured, and only those with glucose levels higher than 225 mg/dl were used in this investigation [16].
Treatment schedule and estimation of fasting blood glucose levels
Animals are divided into five groups: Normal control (NC) rats were in Group 1: STZ (55 mg/kg/s.i.p.), induced diabetic rats were in Group 2: Diabetic control (DC), Group 3: Diabetic rats were induced by STZ (55 mg/kg/b w s.i.p) and treated with a low dose of R. minor stems ethanol extract (200 mg/kg/b w LD/p.o/21 days), Group 4: Rats with diabetes were induced by STZ (55 mg/kg/b w s.i.p) and with high dose of R. minor stems ethanol extract (400 mg/kg b.w HD/p.o/21 days), and Group 5: Rats with diabetes were induced by STZ (55 mg/kg/b w s.i.p) and with Glibenclamide (5mg/kg b.w/p.o/21 days). As per schedule, repeated doses are given for 21 days. As per the guideline’s food and water intake, changes in body weight are analyzed. The blood samples are collected on the 3rd, 7th, 14th, and 21st days to check for body weight and fasting blood glucose levels using a one-touch glucose meter (Accu-check). Within 24 hours of the last dose, blood was collected from rats on an overnight fasting basis from each group by retro-orbital site, to estimate lipid profiles, glycolated heamoglobin, hematological, liver function, kidney function, and enzyme oxidative parameters. Finally, at the end of the twenty-second day, the animals are dissected to isolate the vital organs (pancreas, liver, and kidney) for histopathological examination [17].
Test for oral glucose tolerance
An oral glucose tolerance test (OGTT) was conducted on the 14th day in rats fasting at night. Five groups of rats were formed (n = 6): Group I: was used as the positive control and deionized water, and Groups III and IV were administered RMEE at 200 and 400 mg/kg b. w/p.o, correspondingly. After this treatment, all groups were given 2 g/kg b.w of glucose orally. The posterior vein blood was taken just 30, 60, and 120 minutes after oral administration of glucose [18]. The measurement of blood glucose was done using a one-touch glucose meter (Accu-check).
Body weight
The body weights of the control and treated rats were measured before and three days after the STZ injection (0 days of the experiment). Body weights were recorded on the 7th, 14th, and 21st days [19].
Blood glucose measurement
The collection of blood samples was done from the retro-orbital site of overnight fasting rats and determination of basal blood glucose levels was done before STZ injection and then 3 days after STZ induction (0-day experiment) using the glucose-oxidase method of a glucometer (Accu-check, India) [20]. Blood sugar levels after a fast of control, diabetic, and treated rats were further ascertained every week (7, 14, and 21) intervals [21].
Estimation of hematological and lipid parameters
Hematological parameters like HbA1c, Hb, RBC, and WBC were determined by commercially available kits (diagnostic, Bangalore, India) [22].
Serum lipid profile estimation
After 21 days of therapy, all rats fasted for 12 hours and were thiopental-sodium anesthetized on the 22nd day. After that, the rats were sacrificed via cervical decapitation. The heart puncture resulted in the collection of blood. 10 minutes of centrifugation at 3000 rpm separated the serum. Using commercially available kits (diagnostic, Bangalore, India), the obtained serum lipid profile was analyzed for several biochemical parameters, namely, low-density lipoproteins (VDL-c), very-low-density lipoproteins (VLDL-c) cholesterol, high-density lipoprotein (HDL)-cholesterol [23].
Estimation of liver function and enzyme activities
Following the collection of liver from the animals that were sacrificed, it was independently homogenized in 10 ml of phosphate buffer and at 4°C, centrifuged at 12000 rpm for 30 minutes. The supernatants were collected, and total protein, albumin, SGPT, SGOT, and ALP were estimated using commercially existing reagent kits (diagnostic, Bangalore, India) [24-26].
Estimation of kidney function parameters
After collection, kidneys of the sacrificed animals were homogenized separately in 10 mL of phosphate buffer and centrifuged at 12000 rpm for 30 min at 4 °C. The supernatants were collected, and the concentrations of urea, uric acid, and creatinine were calculated using reagent kits that are commercially available (diagnostic, Bangalore, India) [27-29].
Antioxidant parameters and oxidative stress biomarkers in the liver and kidneys
The anti-oxidant parameters are glutathione (GSH), glutathione-S-transferases (GST), and glutathione reductase (GR), and oxidative stress biomarker malonaldehyde (MAD) enzyme was measured in the liver and the kidney supernatants using commercially available reagent kits (diagnostic, Bangalore, India).
Studies in histopathology
The portions of the pancreas, liver, and kidney separated from the sacrificed rats were studied histopathologically. Tissues were washed in normal saline and quickly fixed for 24 hours in 10%, and then dehydrated with alcohol, moistened with paraffin, cut into slices of 4-5 μm thickness, and stained with hematoxylin-eosin dye for photo microscopic examinations at the 340 µm.
Statistical analysis
The results are reported as Mean ± SEM. The researchers utilized One-Way ANOVA and Dunnett's multiple comparison tests. The GraphPad Prism software (8.4 version) statistical tool was used for all statistical analyses. The significant differences were defined as a p-value of less than 0.0001.
Results and Discussion
Acute toxicity study
In rats, no toxicity was found after R. minor ethanol extract was given at a dose 2000 mg/kg b.w. For this investigation, two dosages of R. minor ethanol extract were chosen: R. minor ethanol extract low dose (LD) 200 mg/kg b.w. and R. minor ethanol extract high dose (HD) 400 mg/kg b.w.
Tolerance test for oral glucose (OGTT)
OGTT was conducted in rats as described in (Figure 1), blood glucose levels increased in the first 30 minutes after glucose injection into normal rats and treated rats, and then the glucose levels fell gradually over the next 60 minutes before returning to normal in the last 120 minutes.
Estimation of blood glucose levels in the fasted state
Compared with the positive control group, blood glucose levels in STZ-induced diabetic rats were statistically (P<0.0001) higher. When diabetic rats were given R. minor ethanol extract in treated groups at a low dose (200 mg/kg), a high dose (400 mg/kg), and standard Glibenclamide for 21 days, fasting blood glucose levels were shown to be statistical (p <0.0001) reduction noticed on the 7th, 14th, and 21st days compared with the diabetic control group (Figure 2).

Bodyweight and consumption of water
Diabetic rats suffered a loss of weight when compared with non-diabetic rats. Gradually increased the body weight of treated groups’ low dose, high dose, and standard groups compared to the diabetic control group. As compared with normal rats, diabetic rats considerably increased their meal and water intake. These signs and symptoms, which are the direct results of inadequate insulin, are well-known indicators of type 2 diabetes in both human and animal models [30]. The body weight of the rat’s R. minor extracts was observed to be growing, probably as a result of the reduced glucose levels, which spared the body fat and muscle protein that would be used in diabetic rats. The end body weights of the diabetes control group were considerably (p <0.001) lower than the normal control group. (Figure 3) shows that administering R. minor ethanol extract at 200 mg/kg b.w. and 400 mg/kg b.w improved body weight statistically (p <0.001) compared with the diabetes control group. The daily water consumption of healthy adults’ rats was 21±5 mL, while diabetic rats consumed 105±5 ml (Figure 4).
Estimation of glycolated heamoglobin and hematological parameters
HbA1c is produced by a non-enzymatic reaction (glycosylation) of glucose and free amino groups of Hb [31]. Hematological measures such as HbA1c higher and Hb, RBC, and WBC were considerably lower in diabetic rats. As compared with the diabetic control, R. minor treated groups at a low dose (200 mg/kg), a high dose (400 mg/kg), and standard Glibenclamide significantly reduced HbA1c and WBC levels (p 0.001). Hb and RBC levels were significantly (p <0.001) when compared with the diabetic control group, with increased total Hb, which might be the result of an improvement in glucose metabolism, as presented in Table 1.

Each bar represents the mean ± standard mean error of the mean (n=3). Diabetic control group versus the normal control group and all treated groups, where all bars are statistically significant from each other (P < 0.0001).
Estimation of serum lipid profile
Concerning lipid parameters, STZ-induced hyperglycemia causes body weight loss due to the increased muscle wasting and tissue protein loss. It is well-known that in uncontrolled type II diabetes mellitus, while the LDL levels increase, the HDL levels decline, contributing to secondary complications [32]. The effect of the treatment groups when compared with the diabetic control group, R. minor ethanol extracts at a low dose (200 mg/kg), R. minor ethanol extracts at a high dose (400 mg/kg), and standard Glibenclamide significantly reduced LDL, and VLDL, and also significantly increased HDL levels, as indicted in Table 2.
Each bar represents the mean ± standard mean error of the mean (n=3). Diabetic control group versus the normal control group and all treated groups, where all bars are statistically significant from each other (P < 0.0001).
Estimation of liver function and enzyme activities
The liver is the body's main metabolic organ, playing a critical role in glucose and cholesterol balance [33]. Biomarkers for the liver toxicity results in treated groups of R. minor ethanol extract low dose (LD) (200 mg/kg), R. minor ethanol extract high dose (HD) (400 mg/kg), and standard Glibenclamide (SD) groups significantly reduced alkaline phosphatases, SGPT, and SGOT levels as compared with the diabetic control group (p<0.001), as demonstrated in Table 3. R. minor ethanol extract significantly increased total protein and albumin levels compared with the diabetic control group (p<0.001).

Each bar represents the mean ± standard mean error of the mean (n=3). Diabetic control group versus the normal control group and all treated groups, where all bars are statistically significant from each other (P < 0.0001).
Estimation of kidney function parameters
Urea is the main metabolic outcome containing nitrogen in protein metabolism. Uric acid is the primary product of purine nucleotides, adenosine, and guanosine. Creatinine is created and released into bodily fluids endogenously, and its clearance is assessed as an indicator of glomerular filtration rate [34]. The current findings suggest that R. minor ethanol extract low dose (200 mg/kg), R. minor ethanol extract high dose (400 mg/kg), and standard Glibenclamide groups significantly reduced urea, uric acid, and creatinine levels compared with the diabetic control group (p<0.001), as presented in (Table 4).
Each bar represents the mean ± standard mean error of the mean (n=3). Diabetic control group versus the normal control group and all treated groups, where all bars are statistically significant from each other (P < 0.0001). Creatinine at the low dose was not significant.
Estimation of liver and kidney anti-oxidant parameters and oxidative stress biomarkers
After 21 days of therapy with the R. minor ethanol extract, all the GR, GST, GSH, and MDA levels were significantly reduced in STZ-induced diabetic rats in the liver and kidney enzymatic oxidative parameters. Compared with Glibenclamide (5 mg/kg) at a higher dose of 400 mg/kg, RMEE displayed a notable (p<0.001) drop in plasma glucose levels and all the parameters near the standard group. When RMEE-treated rats were compared to diabetic rats, anti-oxidants GSH, GST, GR, and the oxidative stress biomarker MAD levels were shown to be considerably lower (p <0.001), as listed in Table 5.
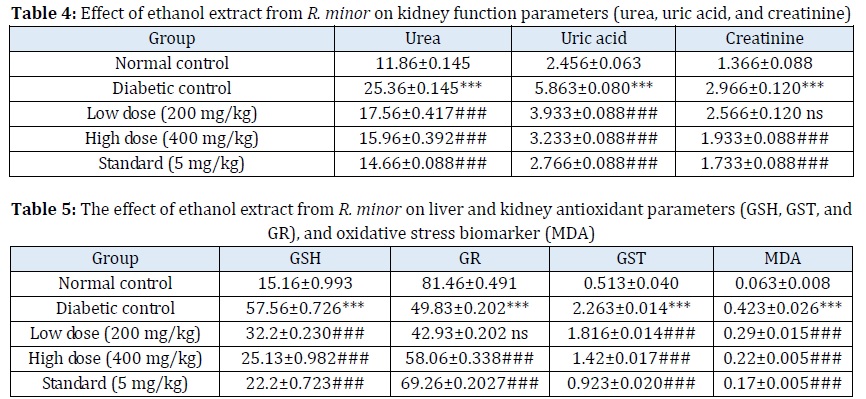
Each bar represents the mean ± standard mean error of the mean (n=3). Diabetic control group versus the normal control group and all treated groups, where all bars are statistically significant from each other (P < 0.0001). GR at the low dose was not significant.
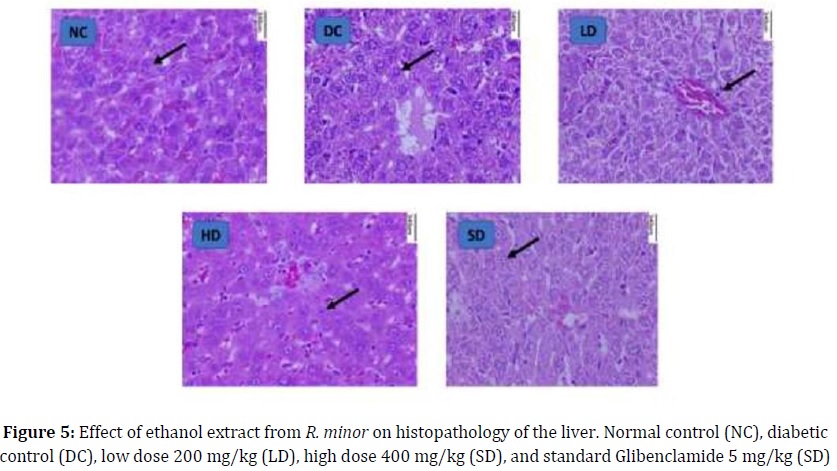
Pathology of liver
The histopathological examination at 340 nm, (Figure 5) of the liver tissues of the negative control (NC) group demonstrated the normal hepatic architecture with central veins and radiating hepatic cords, Kupffer cells, hepatic sinusoids, and polygonal in shape with oval-shaped nuclei in the center and more euchromatin than heterochromatin (Figure 5A). Liver tissue examination in the diabetic control (DC) group revealed widespread hydropic degeneration in hepatocytes and necrosis was observed as a single constituent. Vacuoles large and small, with irregular, partially rounded edges were observed in the cytoplasm of degenerated liver cells. Mild fibrosis, bile duct proliferation, and inflammatory cell infiltration were detected in portal areas (Figure 5B). The liver’s tissues examined in the LD group showed mild vacuolated hepatocytes and mild inflammation and there is the restoration of normal tissue morphology (Figure 5C). Examination of the liver tissues of rats in the HD group revealed intact hepatocytes, hepatic architecture, and the modest hepatocyte vacuolation. The number of dysplastic hepatocytes and necrotic cells was reduced and an advanced pattern of recovery was also observed (Figure 5D). The standard group (SD) revealed hepatocytes of animals treated with standard Glibenclamide showed characteristics that were almost similar to the normal liver group. Moreover, active Kupffer cells and rounded nuclei were also witnessed in (Figure 5E).
Pathology of kidney
Kidney was examined histopathologically at 340 nm (Figure 6) of the negative control (NC) demonstrated normal kidney architecture with the glomeruli presence with intact normal endothelium. Urinary space, blood capillaries, and kidney tubes were intact and appeared normal (Figure 6A). The diabetic control (DC) group revealed leukocyte infiltration, edema exudate, necrotic foci, degeneration of the glomerulus, and focal diffuse tubular degeneration (Figure 6B). The LD group showed relatively healthy glomeruli and tubules, diminished and distorted glomeruli, and dilated tubules (Figure 6C). The HD group revealed intact glomerulus with normal-looking endothelium, urinary capsule, and juxtaposition glomerular apparatus. The urinary space was small, and mild constricted, but no aggregations of cells were observed (Figure 6D). The standard (SD) group reveal led relatively healthy glomerulus with renal appears to be restored and slight degenerative alterations in the tubular wall epithelium with a minimum of interstitial fibrosis records (Figure 6E).
Pathology of pancreas
The pancreas was examined histopathologically at 340 nm. In rats, the death of beta-cells is often triggered by streptozotocin treatment after three days, peaking at three to four weeks [35]. Due to their low quantities of free radical scavenging enzymes, beta cells are more vulnerable to nitric oxide and free radical damage [36]. The negative control (NC) group's (Figure 7) demonstrated the normal histopathological structure of the area of the islet’s beta cells with typical spherical nuclei and extensive granular cytoplasm (Figure 7A). The pancreas of the diabetic control (DC) group was found to be irregularly occupied by a uniform eosinophilic material that was not well defined, and necrosis of the cells could be seen. Langerhans islet cells were shown to have degenerative and necrotic alterations. There was a significant loss of cells in the islets and a disruption of cell order, the blocks were atrophied and the structure degraded (Figure 7B). Examination of the pancreatic tissues of rats in the LD group showed the minimal pathological changes with mild atrophy in Langerhans islets and a small number of degenerations in islet beta cells.


The islets are present with a smaller volume as compared with the normal group. The sinusoids in the bloodstream also appeared normal (Figure 7C). Examination of pancreatic tissues of rats in the HD group showed significant improvement was observed in the Langerhans islet with improvement in the pancreatic islet morphology. This group found that Langerhans islets have a normal beta-cell population (Figure 7D). Examination of the pancreatic tissues of rats in the standard group (SD) showed normal islets of Langerhans with a normal population of beta-cells and an absence of any degenerative changes observed in this group. The islets are well-defined and no necrosis of the cells was observed (Figure 7E).
Conclusion
The ethanol extract of Rourea minor (twigs) may have potential anti-diabetic and antioxidant effects in diabetic rats induced by STZ, and the effect was shown to be more comparable to the drug Glibenclamide. This is the conclusion of our research. Gradually increased body weight. The extracts both dramatically lowered the lipid profile markers and fasting glucose levels in diabetic rats. In diabetic rats, the ethanol extract of Rourea minor was reported to dramatically reduce the SGPT, SGOT, and ALP activities. Rourea minor anti-diabetic and anti-oxidative qualities are strongly suggested by our histological examination, the normal architecture of the liver, kidney, and pancreas in that pancreatic islet, beta cells, endothelium, urinary space, and Kupffer cells were similarly altered in diabetic rats provoked by streptozotocin. The changes were reportedly reversed to near normal after 21 days of oral administration of RMEE, or Glibenclamide, and biochemical analyses. To suggest the proper mechanism for plant anti-diabetic activity, more mechanistic research is necessary.
Acknowledgments
The authors appreciate the help they received from Sri Ramaswamy memorial College of Pharmacy and Sri Ramaswamy Memorial University, Chennai.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
ORCID:
Kavya Yedelli
https://www.orcid.org/0000-0003-4969-5774
Ramachandran Kumar Pathangi
https://www.orcid.org/0000-0002-2737-0365
HOW TO CITE THIS ARTICLE
Kavya Yedelli, Ramachandran Kumar Pathangi. Assessment of Anti-Diabetic and Antioxidant Activities of Rourea Minor Stems in Streptozotocin-Induced Diabetic Rats. J. Med. Chem. Sci., 2023, 6(6) 1370-1382


