Document Type : Original Article
Authors
- Suzan Muslim Abdullah 1
- Abbas Ali Salih AL-Hamdani 2
- Suha Mohamed Ibrahim 3
- Labeeb Ahmed Al-Zubaidi 3
- Farqad Abdullah Rashid 3
1 Department of Chemistry, College of Science for Women, University of Baghdad, Baghdad, Iraq
2 1Department of Chemistry, College of Science for Women, University of Baghdad, Baghdad, Iraq
3 Ministry of Higher Education & Scientific Research & Science and Technology, Directorate of Environment & Water
Abstract
Myrtle plant was washed, dried, and powdered after harvesting to produce a fine powder used in water treatment. An alcoholic extract was created from the myrtle plant using ethanol, which was then analyzed using GC-Mass, Fourier Transform Infrared spectroscopy, and ultraviolet-visible spectroscopy to identify the active components. Zinc nanoparticles were created using alcoholic extract. FT-IR, UV-Vis, SEM, EDX, and TEM were used to characterize zinc nanoparticles. Using a continuous processing procedure, zinc nanoparticles with myrtle extract and powder were employed to clean polluted water containing pesticides and antibiotic. First, 2 g of zinc nanoparticles was mixed with 20 ml of polluted water and the result was (Tetra 44%, Levo 32%), after that used 4 g (Tetra 100%, Levo 100%). Next, myrtle plant was used to treat water (Tetro 100%, Levo 100%). As compared myrtle powder with zinc nanoparticle, it was found that myrtle plant was preferred in water treatment.
Graphical Abstract
Keywords
Introduction
Water is a basis for life; its rate is 71% of the surface level. Despite the essential water rate, the situation is not easy, where the population has increased and their resumption of pure water needs in the time of all activities of the living object to the intention of this importance. There was an urgent need to pure water, particularly in the fields of irrigation or industry, as the sources of clean water over time started to diminish as the demand for it grew [1]. It was crucial to create scientific, practical, and effective ways to treat water because of the growing demand for pure and industrial water, which simultaneously increases water pollution and affects its sources with chemical pollution (both organic and inorganic) [2]. A quantitative and qualitative analysis of pollutants in the water should come before choosing a treatment procedure, and only then the optimal course of action can be decided. There are therapies that use physical techniques, and others that use chemical or biological techniques. However, cost and efficiency are the key factors to consider when comparing various systems [3, 4]. Therefore, researchers looked for the affordable and effective therapy options that would not disturb the delicate balance of the environment. Due to their propensity to absorb chemical contaminants, plant residues are used in water treatment, according to numerous studies. Wastewater has been treated using water duckweed, hyacinth, vetiver grass, and water lettuce. Due to its unchecked development in water bodies, Eichhornia crassipes have been labeled a problematic aquatic free-floating weed. It is also challenging to manage and eradicate this plant from water bodies [5, 6]. On another hand, it has been regarded as a bio indicator because of its capacity to absorb heavy metals from the aquatic habitat. Only few studies have been done on the phytoremediation method of employing Eichhornia crassipes to clean kitchen wastewater. Chemists have been concentrating on nanotechnology in recent years, particularly nanotechnology connected to plants, or "Green Nano" [7, 8]. Another definintion for green nano-method used to integrate plant with chemicals is one of the effective, fast, ineffective, and environmentally friendly modalities. It is common to the methods of usefulness for being a very vital way including multiple plants using the plant in the nut and prevention of nano prepared for the purpose of importance of the environment [9]. In this work, zinc nanoparticle was prepared with myrtle plant and characterized by techniques (FT-IR, UV-Vis) to extract alcoholic myrtle plant and zinc nanoparticle with alcoholic extract with myrtle plant, (SEM, TEM, XRD, and EDX) for zinc nanoparticle. After that, the heavy metals polluted water was treated using zinc nanoparticles with alcoholic extract of myrtle plant and myrtle plant powder and they were compared to find the optimal method. To treat water tainted with phenols and aromatic chemicals, silver nanoparticles have already won the use of extracts utilizing specialized capsule [10]. To purify water of inorganic pollutants, another study combined ZnNPs with alcoholic cumin leaf extract [11].
Materials and Methods
The following chemical substances were employed, and a Shimadzu-3800 Spectro-meter equipped with a (FT-IR) in the range (4000-400) cm-1 was used as a detector: ethanol 20%, methanol 65%, zinc sulphate, ascorbic acid, sodium hydroxide, and polyvinyl alcohol. Shimadzu 160 Spectro-photometer was used to find the electronic spectrum data. Compounds were subjected to mass indication analysis using a GC Mass100P Shimadzu. This study used scanning electron microscopy (SEM) to manage the analysis and describe the size and surface which indicate nanoparticles. The best technique to determine the NPs' morphology is (TEM). A sample is passed through an intense electron beam during this technique for microscopy, and the outcome of the electrons' interactions with the material is a picture creation. A little drop of nanoparticles was applied on a copper grid that had carbon coating, and it was left to dry under a mercury lamp for five minutes. Finally, readings were taken under the stable voltage conditions at magnifications of 5000x, 10000x, 20000x, and 50000x. To validate the presence of the zinc nanoparticle, the EDX measurement was performed using the high-resolution chemical compounds in a sample.
Plant harvest and preparation for grinding and extraction
The used myrtle plants for this study came from the Kadhimiya District in Iraq's Baghdad Governorate. It was thoroughly cleaned with deionized water several times to get rid of any impurities, dried at room temperature, and then grounded into a very fine powder using a special laboratory grinder for 15 seconds. The alcoholic myrtle extract was produced by mixing a solution of 65 % of methanol, 20% of ethanol, and 15% of free ionized water; it was allowed to sit for a while, and then it was heated to 50 °C. It was condensed simultaneously for eight hours using a laboratory condenser. At 50 °C, the extraction procedure is carried out, and then evaporation and condensation are used to boost enrichment, as displayed in Figure 1 (A.myrtle leaves after washing, B.leaves after drying up, and C.myrtle plant after grinding).
Preparation of zinc nanoparticles
The following steps were used to manufacture zinc nanoparticles with myrtle extract, using a modified version of Elumalai's method: 10 mL: 20% of myrtle extract and 1000 ml of pure water were placed in a round flask. The flask was left to be heated and stirred at different temperatures. After one minute, 17.5 g of zinc sulfate was added gradually while being continuously stirred [12]. The mixture's acidity was then equalized by adding pure sodium hydroxide. Thereafter, the mixture was put into a glass container and heated to 200 °C for two hours. The filtered solution was separated from the precipitate to complete the separation, and then the precipitate was collected and dried from the remaining water in A furnace set to 70 °C was used to dry; after it was crashed, 17.5 g of zinc sulfate will be gradually added to finish the mixing of the components. The produced green powder was kept in a container for characterization [13], as depicted in Figure 2 (A. Zinc nanoparticle during preparation, B. Zinc nanoparticle after drying up).

Figure 1: A) Myrtle leaves after wash, B) leaves after dried, C) myrtle plant after grinded

Figure 2: A) Zinc nanoparticle during preparation, B) Zinc nanoparticle after drying up
Determination of the most important compounds of myrtle plant extract
Gas chromatography-mass spectroscopy technique (GC-Mass), which is pure, quicker, and cheap price than the conventional extraction methods, was used to identify the activated compounds in the alcoholic myrtle plant extract. GC-Mass is an analytical technique for a qualitative and quantitative group of a broad domain of compounds [14, 15]. Substances found in the myrtle extract by GC-Mass are presented in Figure 3 and Table 1.
Results and discussion
This section describes the results of treating water contaminated with the inorganic elements including Cd, Sb, Fe, Cr, and Cu either using myrtle plants or with zinc nanoparticles and evaluates the effectiveness of the treatment. The used water treatment technique was the m. processing research contrasting the standards for the standard water adopted by the WHO and treated water.
Ultraviolet visible (UV-Vis)
The UV-Vis spectroscopy is the main part to identify the active compounds in the myrtle plants by determination of the absorptions that visible spectrum cause to the colors of the myrtle plant, which was screened as an alcoholic extract and ZnNP with myrtle extract, and then absorbing peaks in the first one indicate at 307 and 676 nm and in ZnNP 292 nm, this peak refers to the ultraviolet area belong n→ σ*, π→π* at 307 and 676 nm [16]. These peaks refer to (n→π*) and (π→π*) in the UV, 676 nm refers to myrtle plant with both organic and inorganic compounds. As compared both samples, alcoholic-extract peak shifted towards a shorter longer wavelength 10 nm red shift, and another peak in (Vis) area refers to ZnNP [17, 18], as indicated in Figure 4.

Figure 3: GC-mass spectroscopy for myrtle extract


Figure 4: The UV-Vis spectra for two samples
Fourier Transform-Infrared (FT-IR)
Infrared Fourier transform is a key method. When distinguish alcoholic extract with myrtle plant and alcoholic extract with nanocomposite, the method for defining organic components in alcoholic extract refers to the creation of nanoparticles. It is employed to identify the active chemicals and test whether they can be joined with another element to create new complexes. A peak may be seen at 3371 cm-1, and at 3417 cm-1 zinc nanoparticles can be seen. The (O-H) group has altered in two compounds at around 46 cm-1 in these peaks, which are then followed by further peaks on 2929 cm-1 in extract and 2926 cm-1 refer to ZnNPs. These peaks correspond to the (CH) aliphaticgroup (1612 cm-1), the carbonyl group (1552 cm-1), the (CH) group (1037 cm-1 in the myrtle alcohol extract), and the (CH) group (1116 cm-1 in nanoparticle) [19, 20], as demonstrated in Figures 5 and 6.
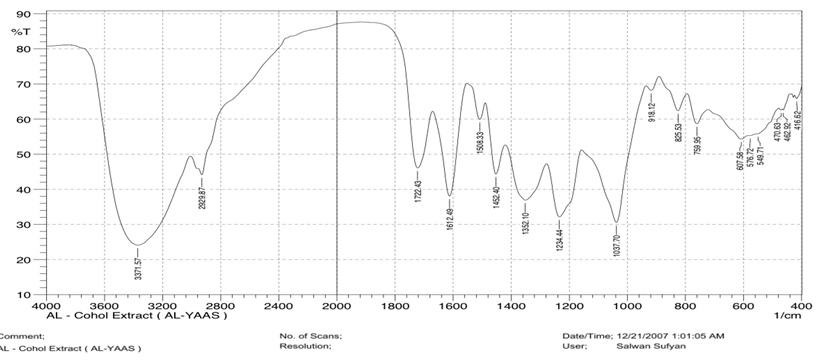
Figure 5: FT-IR spectrum of myrtle alcoholic extract

Figure 6: FT-IR spectrum of zinc nanoparticle with alcoholic myrtle
Scanning Electron Microscope (SEM)
Scanning Electron Microscope for zinc nanoparticles shows spherical crystal shape in the average particles (122.8 nm), which refers to zinc element, this indicates that it has granules size including the nano range [21]. The other nanoparticles appear on (55.96, 63.93, 60.66, 85.32, 97.20, 100.1, and 122.8) nm, which refer to hydrocarbon group range of nano except hydrogen because of its small size (Figure 7) [22].
X-Ray diffraction spectroscopy
Is a technique used to identify the arrangement of the crystal atoms, where the X-Ray hit the crystal to show certain directions according to the angles and strength of reflected rays, where 3D images are formed for the Electrons density inside the crystal. To measure the X-Ray diffraction, the nano-crystals inserted on the angle meter and transformed while targeting the X-Ray on it, which shall result in random diffraction called measurements. All directions image of angles is taken for the 2D dimensions to be transformed to 3D images representing the electronic density inside the crystal. The study of the X-Ray diffraction used for identifying the crystal molecular weight, the result of X-ray testing, where the three readings between were 30-40 due to zinc elements presence in more readings due to the size of nano-molecule with zinc elements, while the remaining values show the presence of hydrocarbon compounds except the hydrogen which does not appear because of its small size [23] as illustrated in Figure 8.

Figure 7: SEM for zinc nanoparticle with alcoholic extract of myrtle

Figure 8: XRD-diffraction of zinc nanoparticles
Energy dispersive X-Ray spectroscopy
EDX is a type of X-ray emission used to identify the chemical properties of the samples, which offers information about the location of atomic distribution on the surface and the chemical compounds of the samples. In other words, each element has an atomic structure and determined values in an X-ray spectroscopy. When examining nanoparticle, it was determined with three peaks (1, 8.6, and 9.8) keV and they were crystal spherical structure [24], as exhibited in Figure 9 (Energy Dispersive of X-Ray spectroscopy (EDX)).
Transmission electron microscopy (TEM)
TEM is the greatest method to figure out how nanoparticles are shaped. A sample is run through a powerful electron beam in this sort of microscopy, and the outcome of the electrons' interactions with the material is the creation of an image. After that, the image is magnified and focused onto an imaging medium, such as a charge-coupled device, a fluorescent screen, or a sheet of photographic film [25] with a smaller magnification. Because of differences in the material's composition or thickness, the contrast can be seen in TEM pictures of that substance [26]. When using a sample of zinc nanoparticles with myrtle extract, the high-resolution transmission electron microscopy (HRTEM) revealed that the sample was smooth and spherical on the outside [26], as shown in Figure 10 TEM of zinc nanoparticles.

Figure 9: Energy dispersive of X-Ray spectroscopy

Figure 10: Transmission electron microscope of zinc nanoparticles
Treatment of organic compounds
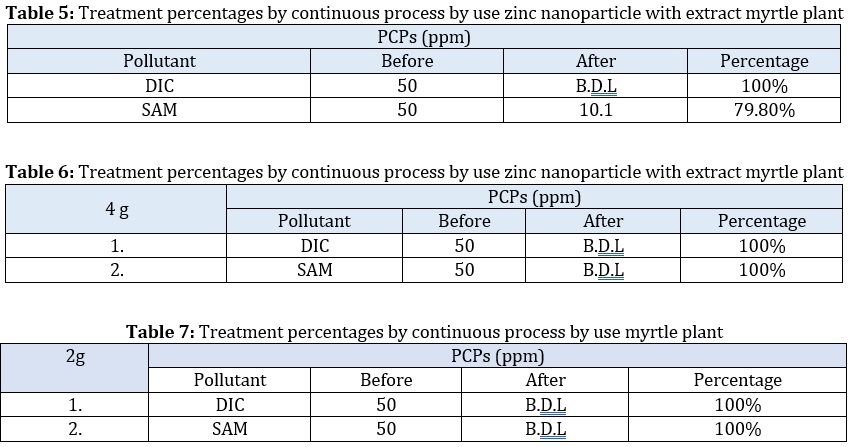
The polluted water was identified using a specific ratio of (Tetra 44%, Levo 32%), (DIC 100%, SAM 100%) 2 g for each sample. Thereafter, a continuous technique of treatment was used. Ten milliliters of polluted water were added along with 2g of myrtle powder and the same method with zinc nanoparticles. After that, the shaking and mixing process was completed by placing the ingredients onto a magnetic stirrer. A portion of the sample was pulled out after the end flow rate, and the treatment % was calculated. The subsequent myrtle powder percentages are 100%. Comparatively, the percentages of the components were found. Because some free active plant groups were found as in GC-Mass in plants in comparison to zinc as there are some copulatives involving with nano zinc or a layer of nano zinc, it reduces their activities as in the following result [27-30], as presented in Tables 2, 3, 4, 5, 6, and 7 and Figures 11, 12, 13 and 14.





Conclusion
In this study, water was treated via environmentally safety methods, such a method accomplished successfully by obeying zinc nanoparticles which was in turn prepared by zinc sulfate as starting material and the formation of which is proved by the following techniques (SEM, EDX, XRD, FTIR, UV, and TEM). Then, the contaminated water was treated from organic and inorganic elements by continuous processing in which zinc nanoparticles were used with alcoholic myrtle extract and myrtle powder alone. It was noticed that myrtle powder was preferred as compared with zinc nanoparticle because some free active plant groups were found as in GC-Mass in plants comparison with zinc as there are some copulatives involving with nano zinc or a layer of nano zinc, it reduces their activity as in the following result, more accurate and more efficient.
Acknowledgments
The authors would like to thank the Department of Chemistry, College of Science for women, Baghdad University, Prof. Dr. Abbas Ali Salih Al-Hamdani, and Ministry of Higher Education, Scientific Research, and Science and Technology.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
The author declared that they have no conflict of interest.
ORCID
Suzan Muslim
https://orcid.org/0000-0002-2073-1324
Abbas Ali Salih
https://orcid.org/0000-0002-2506-986X
HOW TO CITE THIS ARTICLE
Suzan muslim Abdullah, Abbas Ali Salih AL-Hamdani, Suha Mohamed Ibrahim, Labeeb Ahmed Al-Zubaidi, ,Farqad Abdullah Rashid. An Evaluation of Activity of Prepared Zinc Nanoparticles with Extract Green Plant in Treatments of Diclofenac, Levofloxacin, and Tetracycline in Water. J. Med. Chem. Sci., 2023, 6(6) 1323-1335


