Document Type : Original Article
Author
Chemistry Department, College of Education for Girls, Mosul University, Iraq
Abstract
This research presents a rapid and accurate spectrophotometric method for furosemide determination. This method depended on charge transfer reaction of furosemide with pyrogallol reagent using sodium carbonate. It was observed that a product with a bluish-green color was formed after completing the addition and gave the highest absorption intensity at the wavelength of 610 nm. Following Beer's law, the straight standard curve was obtained in the concentration range of (1-20 µg/mL. The statistical results showed that the method has good accuracy and agreement. The molar absorptivity value was 2.3813×104 l/mol.cm and sensitivity of Sandell's was 0.0138 µg/cm2. The relative standard deviation (RSD%) values ranged from 0.18 to 0.71%, relying on the concentration level. For the furosemide estimation, the suggested method has been successfully applied in its pharmaceutical preparations and pure form.
Graphical Abstract
Keywords
Introduction
Furosemide has several chemical names: 4-cliloro-N-(2-furylmethyl-5-sulfamoylanthranilic acid, 4-chloro-N-furfuryl-5-sulfamoylanthranilic acid, 4-chloro-2-furfurylamino-5-sulphamoyl benzoic acid, and 5-(amino sulfonyl)-4-chloro-2-[(2-furanylmethy1) amino] benzoic acid [1]. Furosemide is white crystalline powder, molecular weight (330.7) g/mol, and molecular formula C12H11ClN2O5S soluble in acetone and in dilute solutions of alkali hydroxides, slightly soluble in ethanol, and insoluble in water and methylene chloride [2]. It has the following structural formula (Scheme 1):

Scheme 1: Structural formula of Furosemide
Furosemide has several commercial names, most notably: lasix, Aisemide, Beronald, Desdimin, Frusemide, Fursemide, Lasilix, etc. It is considered a diuretic that prevents the body from absorbing an excessive amount of salt, and this salt is excreted through the urine. Furosemide is mainly used to treat fluid retention in body tissues or edema caused by heart failure, pulmonary edema, and liver and kidney disease; it is particularly effective in treating people with impaired kidney function who do not respond well to thiazide diuretics. It is also used to treat high blood pressure [3-6].
Several techniques and different analytical methods have been used for the furosemide determination such as spectrophotometric methods [7-14], flow injection [15, 16], high-performance liquid chromatography methods [17-19], gas chromatography/mass spectrometry (GC/MS) [20, 21], electrochemical methods [22-24], potentiometric method [25], and atomic absorption spectroscopy [26].
The aim of this study is to develop method rapid, accurate, and simple for furosemide estimation with pyrogallol using charge transfer complex reaction, and also to apply the suggested method to several pharmaceutical formations.
Materials and Methods
Shimadzu UV-Visible-1800 dual beam with identical 1 cm cells was used to perform all spectral and absorbance measurements, Philips PW 9420 pH meter was used for the pH measurements. The chemicals used were all of a high degree of purity.
Working furosemide solution (100 µg/mL)
It was prepared by dissolving (0.01 g) of pure furosemide in 5 mL of ethanol, and then its 100 ml diluted in volumetric flask with distilled water.
Pyrogallol solution (0.1%)
It was prepared by dissolving (0.1 g) pyrogallol with ethanol and completing the volume to 100 ml in volumetric flask with absolute ethanol.
Sodium carbonate solution (0.1 M)
It was prepared weight (1.06 g) of Na2CO3 and dissolved with distilled water in 100 mL of volumetric flask.
Procedure for dosage forms
Three types of furosemide pharmaceutical preparations were used from different companies. Two types were in the form of tablets. They were prepared by weighing 5 tablets after grinding and mixing them well. One tablet (contains 40 mg of furosemide) was dissolved in 5 ml of ethanol and quantity of distilled water, and then the solution was filtered using filter paper and the volume was completed to 100 ml with distilled water. The third type was in the form of a syringe, three injections of (Diasix 20 mg /2 mL Lincoln, Gujarat, India) were taken and 5 mL were withdrawn from it. After that, 5 ml of ethanol was added to it and placed in a volumetric flask of 50 mL capacity and supplemented to the mark with distilled water. Next, the sample solution of three types was prepared by diluting the required volume with distilled water in 100 mL of volumetric flask to obtain a 100 µg/mL solution.
Results and Discussion
Study of optimum reaction conditions
Different conditions and their effects on the intensity of absorption colored solution were studied through the reaction of furosemide with pyrogallol in the aqueous solution.
Study the effect of base type and amount
The effect of different types of strong and weak bases, displayed in Figure 1, was studied by adding fixed quantities (0.5 mL) at a concentration (0.1 M) of each of them separately. Based on the results, it was noted that sodium carbonate is the best to give it the highest absorption intensity. Table 1 indicates that the use of 1 mL volume gave the highest absorption intensity. Therefore, it was used in the subsequent tests.

Figure 1: Types of base

Study the effect of buffer solutions
The pH of the solution was measured before this study and it was found as 4.8. Therefore, the types of different buffer solutions [27] with an acidic function of 4.8 were prepared and their effect on the absorption intensity was studied. Figure 2 illustrates that the use of buffer solutions leads to a decrease in the absorption that’s why they were excluded in subsequent experiments.
Study the amount effect of pyrogallol reagent
The effect of the reagent quantity was studied by taking different volumes (0.25-2.5 mL) of pyrogallol (0.1%) and Table 2 demonstrates that 1 mL of the reagent solution was the best because it gave the highest absorption. Therefore, it was chosen in the subsequent tests.
Study the effect of surfactants
To show the surfactants effect on the absorption intensity of the formed product, different types of surfactants were selected (cetyltrimethylammonium bromide, cetylpyridinium chloride as cationic and sodium dodecyl sulfate as an anionic and non-ionic Triton X-100). It was clear from the results, shown in Figure 3, that their use had a negative effect on the absorption intensity of the product, and thus they were excluded in the subsequent studies.

Figure 2: Buffer solution types

Study the effect of addition sequence
The effect of different sequences was studied to choose the best sequence for the reactants. Based on Table 3, it was noted that the following sequence (S+B+R) gave the highest absorption intensity. Therefore, it continued to be adopted in the subsequent experiments.
Stability of reaction product
The time effect on the absorption of the colored solution was studied with different time periods, and the absorption was measured against the blank solution at 610 nm, as the stability of the colored product for three different amounts of furosemide was studied. The results indicate that the reaction takes place five minutes after completing the additions and is stable with the highest absorption for at least 50 min.
Final absorption spectrum
Under the previously established optimal conditions, the final absorption spectrum of the product formed by reacting furosemide in the presence of sodium carbonate with pyrogallol reagent was studied. The absorption spectrogram showed the highest absorption intensity of the product formed at the wavelength 610 nm, as represented in Figure 4.

Approved working method and standard curve
After fixing the optimal conditions for furosemide determination, the standard curve for the working method was prepared as follows: The increasing volumes of 100 μg.ml-1 from concentration furosemide solution were added. Then, 1 mL of sodium carbonate at a concentration of (0.1 M) and 1 mL of pyrogallol solution at a concentration of (0.1%) were added and diluted with distilled water to 25 mL, and then the absorbance of the solutions was measured at 610 nm against the blank solution. Figure 5 represents the straight standard curve following Beer's law with concentrations ranging from 1.0 to 20 μg/mL. The value of the estimation factor indicates that the linear specifications of the standard curve are excellent. The molar absorptivity was 2.3813×104 l/mol/cm, Sandell's sensitivity was 0.01388 µg/cm2, and also the values of the detection limit (LOD) and the limit of quantification (LOQ) for the method were 0.093 and 0.310 µg/mL, respectively [28].

Figure 5: The standard curve of Furosemide
Accuracy and precision of the method
The accuracy and compatibility of the method were studied, where five replicates were measured for three different concentrations (16, 10, and 4) µg/mL of furosemide solution and treated using the approved method. Based on Table 4, it is clear that this method has good precision and accuracy.
Stoichiometric ratio of complex
The continuous changes "Job's method" [29] is used to find the interaction ratio between the drug compound furosemide and the reagent pyrogallol by following the method of work: A series of volumetric flask containing different volumes of solutions of the equal concentrations 3.023 × 10-4 M were prepared from each furosemide solution (0.5-4.5 mL) and the volumes were supplemented to 5.0 mL of the reagent solution, and then the other solutions were added under the optimal experimental conditions. The absorption of each sample was measured against its blank solution at 610 nm. Figure 6 displays the reaction ratio of furosemide with pyrogallol reagent is (1:1).
Accordingly, the proposed formula for the reaction of furosemide with pyrogallol is as shown in Figure 7 [12].
Interferences study
To examine the method’s selectivity and its application for the pharmaceutical preparations, the effect of the presence of some interactions was studied on the furosemide estimation. The method depended on the addition of different amounts from (100, 500, and 1000) micrograms of the interfering materials to 25 mL volumetric flask containing 4 micrograms of furosemide. It was noted from Table 5 that the studied compounds do not affect the furosemide estimation using the suggested method.


Figure 7: Mechanism of furosemide charge transfer complex formation reaction

Analytical application
The proposed method was applied to the pharmaceutical preparations of furosemide, which were in the form of tablets and injections, and from different origins, by taking three different concentrations of the solutions of the previously mentioned drug preparations. Table 6 represents the success of the suggested method for the furosemide determination in pharmaceutical preparations in the form of tablets and injections. The method had good accuracy and precision.
The proposed method was compared with the standard method approved [2] using t-test and F-test [30]. The results in Table 7 demonstrated that the calculated value of t-test and F-test for five degrees of freedom at a confidence level of 95%. This indicates that there is no variation between the suggested method and the method adopted in the literature, which illustrated the proposed method has a good application for different models of pharmaceutical preparations.
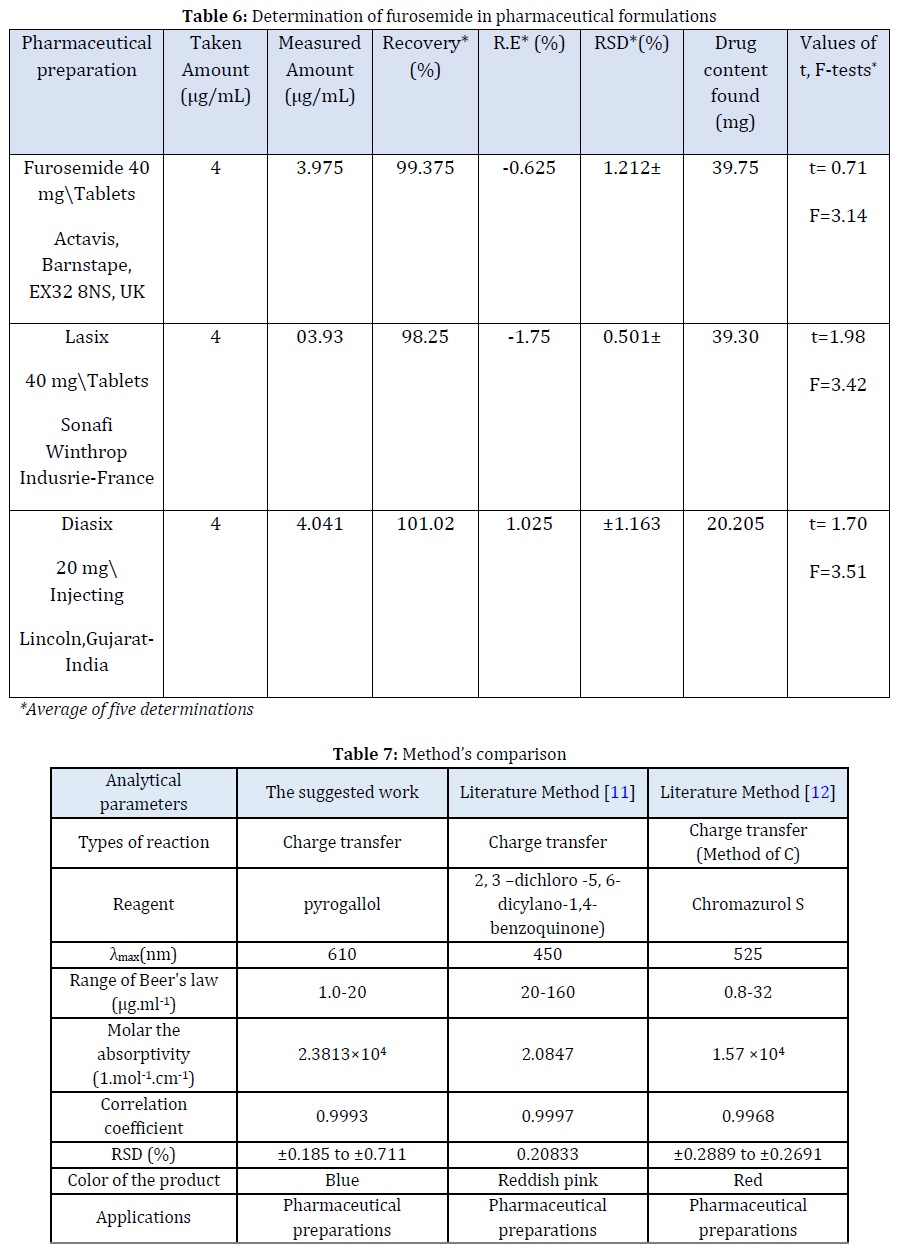
Method’s Comparison
The suggested method has been compared with another spectroscopic method in the literature, and Table 7 indicates this comparison.
Conclusion
The proposed method is fast, easy, and accurate that has been developed for furosemide estimation. The method’s principle depends on the reaction charge transfer complexation between furosemide as donor with pyrogallol reagent as an acceptor in the presence of sodium carbonate form a product of greenish-blue color dissolved in the aqueous medium with the highest absorption at 610 nm. The method follows Beer's law for the range of concentrations 1-20 μg.mL-1. The statistical results showed that this method has good accuracy and precision. The method has good sensitivity, as it does not need use the organic solvents, solvent extraction process, or does not require temperature control. The method succeeded in estimating furosemide in more than one drug.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
The author declared that they have no conflict of interest.
ORCID:
Hind Ahmed Mahmoud
https://orcid.org/0000-0002-3358-9822
HOW TO CITE THIS ARTICLE
Hind Ahmed Mahmoud. Spectrophotometric Determination of Furosemide Using Pyrogallol Reagent in Pharmaceutical Preparations. J. Med. Chem. Sci., 2023, 6(6) 1254-1264
DOI: 10.26655/JMCHEMSCI.2026.6.6


