Document Type : Original Article
Authors
1 Department of Pharmaceutical Chemistry, College of Pharmacy, University of Al-Qadisiyah, Diwaniyah, Iraq
2 Department of Clinical Laboratory Sciences, College of Pharmacy, University of Al-Qadisiyah, Diwaniyah, Iraq
Abstract
The new diazo ligand, and its complexes with metals ions Cu(II), Zn(II), Cd(II), and Ag(I), were synthesized, and ligand synthesis was done via diazotization of 4,4'-Methylenedianiline (MDA), and then coupling was carried out with aniline derivative in good yields by using spectroscopic techniques such as FT-IR, UV-Visible, NMR, and elemental analysis for characterization. Also, the analytical measurements were done such as the detection of conditions of the optimal reaction (reagent concentration, pH, etc.), the detection limit, linearity, and sensitivity. Moreover, the biological activity of the synthesized diazo ligand and its complexes were tested against four types of bacteria (Staphylococcus aureus, Enterococcus feacalis, E.coli, and Pseudomonas aeroginosa,) in vitro. They showed the promising biological activity toward these examined organisms.
Graphical Abstract
Keywords
Introduction
One of the most organic toxic chemicals is aniline. Aniline is used to synthesize numerous organic chemicals such as azo dyes, antioxidants, antiseptics, corrosion inhibitors, insecticides, pharmaceuticals, fuel additives, and rubber, due to dyestuffs and their high toxicity due to the aniline content in wastewater from pharmaceutical manufacturers affects the aquatic ecosystems. Aniline derivatives contribute to the production of pharmaceuticals, pigments, paints, herbicides, rubber, dyes, and polyurethane precursors. Although aniline compounds generated in some pharmaceutical companies are organic, their composition is extremely complicated, resulting in a high level of biological resistance. On the other hand, 4,4-Methylenedianiline has a wide range of applications, including polyurethane spume, cell strengthening in lubricating oils, azo dye manufacturing, iron corrosion protection, and epoxy resin curing agent [1-4].
MDA (4,4-Methylenedianiline) has the potential to damage human and animal health because it causes eye irritation and damages the kidneys, liver, and skin; yet, it has been used to create several Schiff bases and azo ligands with important medical and therapeutic properties [5].
Many strains of organisms exhibit resistance to the conventional antimicrobial treatments, and thus microbial resistance is considered to be one of the most important public health challenges in the world. As a consequence, it has become a major difficulty in health care delivery. The azo compounds have not received much attention in the search for more effective drugs than the current antimicrobials [6]. We report the synthesis of a new azo ligand and some of its complexes to improve the biological activity against some types of bacteria and lower the toxicity of aniline and MDA moieties. The spectroscopic methods (UV-visible infrared (IR), and 1H NMR) were used to investigate their structures. The antimicrobial effects of the ligand and its complexes were also tested in vitro.
Martials and Methods
The infrared FT-IR (Shimadzu, Japan) was used for employ the IR spectra of the compounds. A reference KBris used to compare the study measurements and reference values. A UV-visible spectrophotometer (double beam Shimadzu 1800) was used for the UV-visible study of the complexes. The NMR spectrometer (VARIAN INOVA, 400 MHz, Dmso-d6, TMS, 25 °C) was used to determine 1H-NMR spectrum of ligand. (SMP3/Stuart, UK) was used to measure melting point of the samples.
Reagents and solutions
4-Bromo aniline and 4,4'-methylenedianiline were purchased from Hopkin & Willams and Sigma Aldrich, respectively. All of the other reagents and substances utilized in this research were of the analytical grade, and were used without further purification. They included hydrochloric acid and sodium nitrite from BDH, and absolute ethanol was obtained from (GCC, England). Solutions of 0.0242 g of CuCl2.6H2O, BDH, 0.0183 g of CdCl2, Merck, 0.0170 g of AgNO3, Merk, and 0.0136 g of ZnCl2, Merk in an appropriate amount of deionized distilled water and made to 100 mL with water were prepared as a stock solution with concentration of (0.001 M). Acetate buffer solution (0.1 mol. L–1) (solution of acetic acid and sodium acetate) was used for the synthesis of complexes.
Synthesis of ligand, 2,2'-((methylenebis(4,1-phenylene))bis(diazene-2,1-diyl))bis(4-bromoaniline)(L)
Azo ligand was synthesized according to the literature procedure [7] with some modifications, 4,4'-methylenedianiline (1.3 mmol, 0.26 g) was added to the solution (distilled water 10 mL, HCl 2 mL) under 5 °C with NaNO2 (1 mmol, 0.069 g) in distilled water (10 mL). After 20 min, the producing diazonium salt was mixed with 4-Bromo aniline (2 mmol, 0.22 g) which contained 15 mL of ethanol and NaOH (10 mL, 10%), and then it was kept at an ice-bath 5- 0 °C. The orange colour was formed, and then the product was left stable for 60 minutes and pH was regulated to 6. After that, the solid precipitate was kept for 24 hours, and then it was filtered to get solids. Followed by washing many times by using distilled water, it was dried to produce the ligand and had the orange colour, and then purified by recrystallization from ethanol, yield 78% and m.p 204-207. The synthesis of ligand is given in Scheme 1.
Azo metal complexes preparation
Complexes were synthesized according to the literature procedure [8] with some modification, Ag(I), Cu(II), Cd(II), and Zn(II) (0.005 mol) in (20 mL) buffer solution were added dropwise with shaking to (0.01 mol, 3.06 g) of ligand dissolved in 50 mL of absolute ethanol. The contents were shaken for 15 minutes at 25 °C before being left for 24 hours. The mixture was filtered, washed with ethanol and D.W., and dried in an oven at 60 °C. Scheme 2 exhibits the proposed chemical structure of complexes.
Results and Discussion
All of the complexes and the ligand were found to be stable and coloured at room temperature. As compared with the ligand, complexes exhibited a high thermal stability due to their high melting point. Their solubility is indicated in DMF, DMSO, and ethanol, and also their partial solubility is illustrated in acetone and water. Table l presents the physical properties, of ligand and complexes.
NMR spectra of ligand
The 1H-NMR and 13C-NMR spectra of the ligand was estimated in DMSO-d6 as a solvent with (TMS) as an inside reference (400 MHz). The 1H-NMR spectrum (Figure 1) showed the characteristic single signal at δ 3.9 ppm which are attributed to H2C protons [9]. The appearance of a multiple signal of the aromatic benzene protons of the whole compound appeared at the range 6.50-8.29 ppm [10]. Also, the recorded abroad peak at δ 5.24 is attributed to the NH2 protons [11].

Scheme 1: Synthesis of diazo ligand 2,2'-((methylenebis(4,l-phenylene))bis(diazene-2,l-diyl))bis(4-bromoaniline)

Scheme 2: The metal-ligand structures

The 13C-NMR spectrum of the ligand exhibited a characteristic signal at δ 40.5 ppm due to C13 (CH2), and also it indicated many signals attributed to the aromatic carbon atoms (δ 101.5 for C14,15, δ 110.24 for C18, 24, δ 117 for C20,22, δ 121 for C5,7, δ 129 for C2,10, δ 132 for C21,25, δ 140 for C6,12, δ 147 for C19,23, δ 153 for C4,17, and δ 157 for C16,17 [12-14]. The ligand spectrum is illustrated in Figure 2.

Figure 1: 1H-NMR spectra of ligand

Figure 2: 1H NMR spectra of ligand
Infrared spectra
The infrared studies of L and its complexes were recorded 4000-400 cm-1 with KBr pellets, the ligand spectrum in Figure 3 demonstrates the characteristic symmetric and asymmetric stretching bands at 3409 and 3440 cm−1 due to NH2, respectively [15], the bending band at 1650 cm-1 of the amino group was also observed. Likewise, the spectra showed, a band at 1434 cm−l assigned to azo group [16], while the absorption bands were found at 2923 and 3024 cm-l assigned to C-H aliphatic and C-H aromatic, respectively [17]. A group of bands at 1596-1504 cm-1 was attributed to the vibration of C═C aromatic.
The comparison of the FT-IR spectra of free L with its metal chelates is presented in Table 2 points to the ligand that was principally coordinated to the metals. Through the spectrum of complexes in Figures 4, 5 and 6, it is noticed that the ligand spectrum showed the absence of υ (N-H) band, which appears clearly in all spectra of its complexes at absorption vibration 3348-3425 cm-1. Furthermore, the bending vibration of the amino group at 1650 cm-1 of free ligand was shifted to a lower frequency of 1620-1604 cm-l in the spectra of all complexes [18-21] demonstrating that the amino group is coordinated with the metal ions.
Another indication of coordination is that the (N=N) absorption band of free ligand at 1434 cm-l shifts to 1386-1488 cm-1 in complexes, indicating that azo nitrogen is involved in coordinating with the metal. Moreover, the metal-ligand vibrational frequencies of new bands (M-N) discovered in the spectra of all the complexes were determined in the range of 401-563 cm-1 [22-29].

Figure 3: FT-IR spectrum of the ligand
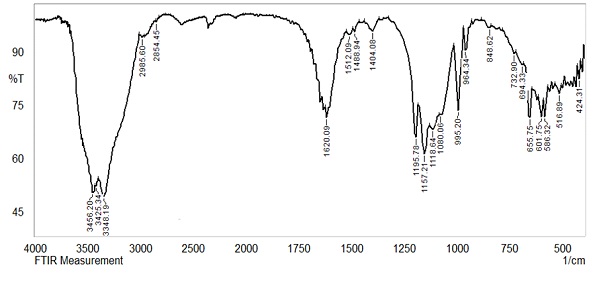
Figure 4: FT-IR spectrum for Cu-L complex

Figure 5: FT-IR spectrum for Cd-L complex

Figure 6: FT-IR-Spectrum for Zn-L complex
Table 2: FT-IR spectra data, cm-l
|
Compound |
υ NH2 |
υ N=N |
υ NH |
M-N(NH), M-N(N=N)υ |
|
C25H20Br2N6 (Ligand) |
3409, 3440 |
1434 |
- |
- |
|
[Cu(L)2] |
3425, 3456 |
1488 |
3348 |
516, 424 |
|
[Cd(L)2] |
3525, 3456 |
1450 |
3410 |
563, 416 |
|
[Zn(L)2] |
3587, 3486 |
1450 |
3425 |
501, 401 |
|
[Ag(L)] |
3405, 3440 |
1386 |
3386 |
524, 478 |
Selection optimal concentration for the reagent
Different concentrations for the reagent were investigated to determine the optimal concentration. It was found that 0.5× 10− 5 M was fairly enough for complexes, as depicted in Figure 7.
Absorption spectra of the synthesized compounds
In absolute ethanol, the electronic spectra of the ligand under study were run. A comparable solvent was used to demonstrate the ultraviolet-visible of the ligand and its chelates (Figure 8) in a concentration of (0.5×10-5 M) at 25 °C from (200-1000 nm). A free ligand revealed large bands at 360 and 289 nm in the UV-visible spectrum. For the intra-ligand electronic transition n⟶π∗, the first band was designated. The following electronic transition broadband, located at (289 nm) was designated to electronic transition π⟶π∗ [30-32].
For electronic spectrum research, the colours that result from the reaction of metal ions with azo ligands are quite significant. The presence of a strong and high absorption peak belongs to the complex. This peak was moved toward the visible region of the complex spectra with respect to the ligand. This might be a result of the complex's coordination and complex formation.

Figure 7: Absorbance spectra for the reagent in different concentration

Figure 8: Absorption spectra a) Reagent = 0.5 × 10-5 M, b) Cu(II),complex, c)Zn(II) complex, d) Cd(II) complex, and e) Ag(I) complex
Method validation
The curves were created by graphing the absorbance signal against each analyte concentration under the typical conditions. The solutions were placed in the optical cell (10 mm) of the metal ion spectrophotometrically opposite the blank created under the same circumstances, as described in Table 3.
The antibacterial efficiency of complexes and ligand
The effect of the synthesized complexes (Ag(L)2, Cu(L)2, Zn(L)2, and Cd(L)2) and ligand were tested on Gram-positive, (Staphylococcus aureus and Enterococcus feacalis) and Gram-negative bacteria (E.coli and Pseudomonas aeroginosa) in nutrient agar by diffusion method (100 ppm) concentration of solutions was prepared by dissolving the synthesized compounds in DMSO solvent. The complexes activity was recorded by measuring the inhibition diameter of the bacterial growth in millimeter unit. The results of inhibition zones are presented in Table 4 and Figure 9.
Inhibition zone (in mm)
The present data indicated that all bacteria under investigation do not have any susceptibility to the Zn complex unlike the Cd complex which displayed an excellent activity with all types of bacteria and more than other complexes. Therefore, ligand has comparatively less efficiency than complexes toward tested microorganisms.
The cause of these results may be due to the formation of hydrogen bond between the examined compounds and the organism cells, and thus affects entirely the cell functions. Furthermore, the presence of metallic sites is a contributing factor in the examined metal chelates and has the stronger antibacterial effects.

Table 4: The antibacterial efficiency of ligand and their metal complexes on Gam-positive and Gram-negative bacteria


Figure 9: The inhibition zones of bacteria by the effects of (1=Ag, 2=Cu,3=Cd, 4= solvent, 5 = ligand, and 6=Zn)
Conclusion
In this article, a new diazo ligand; 2,2'-((methylenebis(4,l-phenylene))bis(diazene-2,l-diyl))bis(4-bromoaniline) was synthesized and treated metal salts to yield the corresponding complexes (Cu(II), Ag(II), Cd(II), and Zn(II). The spectral and analytical methods (IR, NMR, UV-Visible, elemental analysis, and electronic absorption) were used to characterize them. Coordinating of the above mentioned ligand with metals occurs by N atom of azo and N of amine groups. The biological efficiency of the synthesized metal complexes and ligand was examined against four types of bacteria (Staphylococcus aureus, Enterococcus feacalis, E.coli, and Pseudomonas aeroginosa). The results show that ligand and complexes have a good activity against the above-mentioned organisms.
Acknowledgements
The authors would like to thank the University of Al-Qadisiyah, College of Education for providing facilities that improved the quality of this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
The author declared that they have no conflict of interest.
ORCID:
Lina Saadi
https://orcid.org/0000-0002-9524-2218
AzharGhali
https://orcid.org/0000-0002-3480-6040
HOW TO CITE THIS ARTICLE
Lina Saadi, Saba A. Ali, Azhar A. Ghali. Synthesis and Identification of Some Metal Complexes Derived from Azo Ligand of 4,4'-Methylenedianiline and 4-Bromoaniline and Antimicrobial Studies. J. Med. Chem. Sci., 2023, 6(4) 720-732


