Document Type : Original Article
Authors
1 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Kufa, Al-Najaf, 54001, Iraq
2 Department of Chemistry, Faculty of Education for Girls, University of Kufa, Al-Najaf, 54001, Iraq
3 Directorate of Education in Najaf Governorate, Al-Najaf, 54001, Iraq
Abstract
Compact method of liquid ion exchange and cloud point extraction (CPE) was used as a sensitive green method for separating Aluminum(III) as ion pair association complex between Janus green B as a large organic cation with Al(III) as metal anion complex (AlCl4-) from NaCl medium, the optimum conditions were studied, and for the higher extraction efficiency shows a method of extraction needed 0.5 M NaCl in aqueous solution with 100 μg/10 mL of Al(III) ion. 0.5 mL non-ionic surfactant TritonX-100 and heating at 80 °C in an electrostatic water bath for 20 min as a heating time to form cloud point layer (CPL) as the addition phase to extract ion pair association complex formed in the aqueous phase. Thermodynamic data for extraction Al(III) equal to ΔHex=92.530 kJmol-1, ΔGex=-63.263 kJmol-1, ΔSex=441.339 Jmol-1K-1. Likewise, this study included the effect of electrolytes, interferences, kind of surfactant, and kind of organic reagent as in stoichiometry studies. This method is applied for spectrophotometric determining for A(III) in dissimilar samples.
Graphical Abstract
Keywords
Introduction
Al is the third most plentiful element in the Earth's crust and the most abundant metal. It has a high affinity for oxygen [1, 2]. Al is found in high concentrations, but has no recognized biological purpose. In light of Al's abundance in nature and commerce, its relatively low toxicity despite its health consequences is of interest. Deposition in bone and the central nervous system has been linked to toxicity [3, 4]. Furthermore, Al up regulates the gene expression of estrogen signaling in human breast cancer cells [5].
Al concentrations in various samples have been determined by using numerous analytical methods [6-10], including atomic absorption spectroscopy (AAS), voltammetry, inductively coupled plasma (ICP), mass spectrometry, and flow injection. The spectrophotometric methods are popular because they are easy to use, quick, cheap, and have numerous applications [11-15].
By using the crown ether 15C5 as a complexing agent, Al(III) was successfully recovered as the chloroanion complex AlCl4- from the NaCl medium. Shaking time, the influence of organic solvents, and the cavity size was also investigated, as were the amounts of NaCl and Al(III) ions. Thermodynamic evidence indicates that the ion exchange reaction was exothermic. The adopted stoichiometry for the extracted species has been 1:1:1 [Na15C5]+; AlCl4-.The extracted complex is based on λmax=241 nm. The environmental and vital projected method for a spectrophotometric determination of Al(III) in dissimilar samples, D.L= 1.78×10-5 ppm, ε= 300.191 L.mol-1.cm-1, Sandell’s sensitivity= 0.00899 μg/cm2 [16].
Materials and Methods
Apparatus
Absorbance readings were taken with a Biochrom Libra S60 UV-Vis spectrophotometer from the United Kingdom, an English electrostatic water bath (Haburg-90).
Chemicals
Stock solution 1000 μg/mL for Al(III) was prepared by dissolving 1.27 g of AlCl3.6H2O in 100 mL aqueous solution containing 0.5 mL of HCl by using a volumetric flask. Also, the standard solution of Janus green B at 1×10-2 M was prepared by dissolved 0.0511 g in 10 mL distilled water by using a volumetric flask. Dilution with purified water yielded more workable solutions.
Samples collect and preparation
Polyethylene bags were used to store soil samples taken at 15-centimeter depth. Oven drying takes 8 hours for soil samples at 80 °C [17]. The water sample was kept in polypropylene flasks in the refrigerator [18]. To create various sample solutions, 5 g of sample was combined with 10 mL of HNO3. A 2-3 mL volume reduction was achieved by heating the solution on an electric hotplate. The solution was cooled, and then heated to boiling with an additional 10 mL of concentrated HNO3, 5 mL of concentrated H2SO4, and 4 mL of concentrated H2O2, until the volume decreased to 2-3 mL. Finally, 10 mL of water was added until a colorless solution was obtained, indicating the complete oxidation of the organic matter. After being filtered through filter paper and chilled, the solution was transferred to a 100 mL volumetric flask and diluted with distilled water until it reached the correct concentration [19].
General extraction method
Making up liquids from water Cloud point layer (CPL) is formed when a solution containing 100 g of Al(III) ions and 0.5 M NaCl is heated in an electrostatic water bath at 80 °C for 20 minutes, after which the CPL is separated from the aqueous solution, dissolved in 5 mL ethanol, and its absorbance at λmaxmax=652 nm is measured against a blank prepared in the same way but without Al(II) ions. Al(III) ion concentrations in the extracted aqueous solution were determined by using the 8-hydroxy quinoline spectrophotometric method [20] by referring back to the calibration curve in Figure 1.
By using this connection, we can determine the distribution ratio D as an extraction efficiency metric by subtracting the amount of Al(III) ions delivered to the (JGB) ion pair association complex.

Results and Discussion
Spectroscopic study
In Figure 2, the UV-Vis spectrum is observed for the ion pair association complex of Al(III) ion as anion AlCl4- with JGB, demonstrating the greatest absorbance of the ion pair association complex extracted into CPL occurred at a wavelength of λmax=652 nm.
Effect of NaCl concentration
Making a Water-Based Series Solution Heat, these solutions were kept in an electrostatic water bath at a suitable temperature and time until a cloud point layer CPL is formed as a general method. The results are as indicated in Figures 3F3 and 4: 10 mL volume, 100 g Al(III) ion, increasing NaCl concentration, 1×10-5 MJGB, and 0.5 mL of surfactant Triton X-100.

Figure 1: In aqueous solutions, a calibration curve for A(III) by using the 8-hydroxy quinoline technique

Figure 2: The visible and ultraviolet light spectra of an ion pair interaction complex for Al(III) as AlCl4- with JGB

Figure 3: The consequence for NaCl concentration on forming and stability of ion pair complex of Al(III) ion

Figure 4: The consequence for NaCl concentration on extraction efficiency of Al(III) and D-values
The results reveal that 0.5 M NaCl gave the highest extraction efficiency for Al(III) ions, while also providing the best thermodynamic equilibrium for forming AlCl4- so that the ion pair association complex with JGB could be extracted to CPL. This is important to keep in mind because any concentration of NaCl below the optimum would not allow for thermodynamic equilibrium to be reached. In contrast, any concentration above the optimum would result in a decrease in extraction efficiency.

Effect of triton X-100 volume
100 μg Al(III) ion was extracted in 10 mL aqueous solution in the presence of 0.5M NaCl, 1×10-4 M JGB. with different volumes of Triton X-100 according to the general extraction method. The consequences are depicted in Figures 5 and 6.

Figure 5: Result of surfactant volume on the formation of good CPL for extraction complex

Figure 6: Outcome of surfactant volume on extraction efficiency of Al(III) ion and D values
The findings demonstrate that 0.5 mL of TritonX-100 was the best amount because it gave the highest extraction efficiency for Al(III) ions. This amount of surfactant also gives the state of critical micelles concentration (CMC), which gives the best aggregation for micelles to form the best CPL with suitable properties to show the best extraction efficiency. Any amount of TritonX-100 less than the optimum value was not enough to reach CMC. Hence, the extraction efficiency went down.
Effect of temperature
After heating all the solutions in an electrostatic water bath for fixed time at different temperatures, the results are shown in Figures 7 and 8.
After that, we figured out the constant extraction Kex at each temperature by using the following application relation. The results are demonstrated in Figure 9. Also calculated all thermodynamic data, as in Table 1.


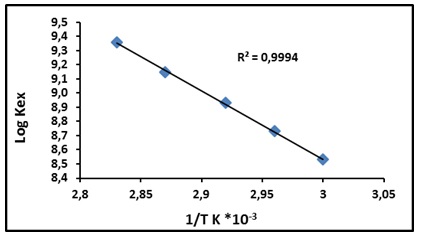
Figure 9: Consequence for temperature on extraction constant
Table 1: Thermodynamic data for extraction of Al(III) ion

The results demonstrate the optimum temperature, which gives a higher extraction efficiency of Al(III) ion according to the compact method of liquid ion exchanging with cloud point extracting was 80 °C. This temperature provides the necessary energy for suitable aggregation micelles of surfactant to form CPL to extracted complex produced between Al(III) ion and JGB quantitatively so that the low value of enthalpy of extraction ΔHex indicates the great convergence for ion pair complex extracted to CPL, besides the sizeable positive magnitude of ΔSex indicates the extraction method rely on entropy to form ion pair complex extracted, and that is mean the extraction method was entropic in the region.
Effect of heating time
100 μg Al(III) was extracted in series aqueous solutions 10 mL in volume according to general method at the optimum conditions when these solutions were heated in an electric water bath at 80 °C for different periods. The results are represented in Figures 10 and 11.

The results showed that 20 minutes of shaking was the best time to get the most out of the extraction method. The heating time represents the kinetic side of the extraction method, which helps to complete the micelles aggregation to form a better CPL. The heating time longer than the optimum value decreases the extraction efficiency by making micelles move around more.
Effect of chloride salt kind
Al(III) ion was extracted at the best conditions by using the general extraction method in the presence of different chloride salts. After measuring the absorbance of the ion pair association complex was extracted into CPL and the distribution ratio D was figured out for each chloride salt. The results are displayed in Figures 12 and 13.
The results reveal that KCl was the best chloride salt for ion pair association complex formation and higher extraction efficiency. This is because each chloride salt in the aqueous phase took part in the complexes formation that was extracted by forming aluminum anion complexes AlCl4- with high concentration and stability.
Effect of surfactant kind
Al(III) ion was extracted by using the general extraction method under the optimal conditions with the presence of different surfactants. The results are presented in Table 2.

Table 2: The effect of surfactant kind on extraction efficiency

The results demonstrate that TritonX-100 was the best surfactant. This is because the structure of the surfactant micelles made the best CPL, which could separate the higher concentration of Al(III) ions that formed in the aqueous phase. This means that the extraction efficiency was high.
Stoichiometry
To pinpoint a more probable structure of ion pair association complex, extracted followed to spectrophotometric method, slope ratio method, and slope analysis method. The results are depicted in Figures 14 and 15. Distribution ratio (D) slope analysis method is depicted in Figure 16.
Based on the slope ratio and slope analysis, it was found that the most likely structure of the ion pair association complex was 1:1:1.



Figure 16: Distribution ratio (D) slope analysis method
Effect of Interferences
Al(III) ion was extracted by using the general extraction method under the best conditions, while 0.01 M of foreign metal cations were presented. The results are indicated in Table 3.
The results show a decline in extraction efficiency of Al(III) ion in the presence of foreign metal cations that is mean interferences effect due to foreign participation ions Al(III) ion in extraction method, formation ion pair association complex, and this behavior lead to the consumption of some JGB and chloride ion Cl- and decline its concentration from the optimum which necessary to extraction Al(III) ion appear to decrease in extraction efficiency of Al(III) ion.
Effect of organic reagent
According to the general method, the extracted Al(III) ion by using different organic reagents after spectrophotometric study and measuring absorbances and D values, the results were as in Table 4 and Figures 17 to 19.



Figure 19: Al(III)-bright green ion pair association complex UV-Vis spectra
In addition to the fact that different organic reagents work best under different conditions, the data reveal that the extraction efficiency varies depending on which organic reagent is used.
Calibration curve of Al(III)
Preparing a calibration curve by following the extraction method under the optimal conditions for solutions containing growing quantities of Al(III) ion allows for the Al(III) determination in various samples under a general extraction method in the range 0.5-10.0 ppm were obtained by plotting absorbance vs. Al(III) concentration in ppm the straight-line equation y = 0.0178x + 0.0367, R² = 0.9983, ɛ=0.479×103 L. mol-1.cm-1. The LOD and LOQ for Al(III) were obtained at 0.8791 and 2.9302 ppm, respectively. The RSD% (n=3) was 2.943%. The results are demonstrated in Figure 20.
Al(III) concentrations in a range of samples were quantified by using the proposed technique. The atomic absorption spectroscopy was also used to investigate the samples for comparison. Table 5 displays the findings (5). The t-test with a 95% confidence interval demonstrates that the outcomes from both approaches are comparable (tcritical = 2.037, tcalculate = 0.00042). There is not significant between two methods.

Figure 20: Calibration curve for spectrophotometric determination for Al(III)
Table 5: Determination for Al(III) in dissimilar samples through two methods

Conclusion
The proposed method is based on the complexation of Janus green B as a large organic cation with Al(III) as a metal anion complex (AlCl4-) from a NaCl medium developed in the presence of a non-ionic surfactant. High levels of sensitivity, precision, and accuracy characterize the developed approach. When compared with the AAS method, the results were obtained by using the proposed method for determining Al(III) in various samples were found to be quite excellent.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
The author declared that they have no conflict of interest.
HOW TO CITE THIS ARTICLE
Sahar Aqeel Hussain, Safa Majeed Hameed, Ahmed S. Abed, Noura Salih Mahdi. Separation, Pre-concentration, and Determination of Al(III) by Cloud Point Extraction as a Compact Method with Liquid Ion Exchange. J. Med. Chem. Sci., 2023, 6(3) 656-667


