Document Type : Original Article
Authors
Department of Biology, College of Science, University of Misan, Misan, Iraq
Abstract
This study was designed to compare between anti-depressants (amitriptyline and escitalopram) in terms of their impacts on sperm concentration, motility, and vitality, as well as their effect on the hormone level (FSH, LH, and testosterone). The histological sections of the testis and epididymis were stained by using the (PAS) method and the number of experimental 90 male mice was divided into three groups, of which one consisting of 30 male mice, the first group was administration orally normal saline, the second group was administration orally amitriptyline, and the third group was administration orally escitalopram at a dose (0.4 µl/day) twice a day, and continued to experience six weeks, and the results showed a decrease in the concentration and the sperm motility, and also an increase in the number of the dead sperm showed a decrease in hormone levels (FSH, LH, and testosterone) as well as the interaction of the basement membrane of testis and epididymis with PAS ranged from moderate to strong in both groups.
Graphical Abstract
Keywords
Main Subjects
Introduction
Antidepressants are known as those medications that alter the chemical disturbances of neurotransmitters in the brain, thus helping to detract symptoms of depressive disorders and that chemical imbalances lead to changes in mood and behavior, neurons in the brain communicate through neurotransmitters, the vesicles in neurons contain the neurotransmitters by which neurons communicate with each other, neurotransmitters such as serotonin, dopamine, and noradrenaline or norepinephrine are released from the outer end of one nerve and received from the other [1]. Amitriptyline is intended for the treatment of various neuropathies, migraine prophylaxis, and fibromyalgia [2, 3]. It acts as inhibiting the uptake of serotonin and norepinephrine, and thus intensifies the neurotransmitters in the synapses [4]. Escitalopram is an antidepressant of the SSRIs class [5]. This type acts according to the mechanism of stimulating post-synaptic receptors by blocking the serotonin uptake in neurons (pre-synaptic), and thus increasing the concentration in the synaptic space [6].
Many factors negatively affect the male reproductive system, and thus cause infertility, including sexual factors, congenital anomalies, and endocrine disorders, in addition to radiation, climate, and drugs [7]. Hormones (FSH, LH, and testosterone) are used as markers for spermatogenesis and the testis activity in males [8]. FSH is a glycoprotein that participates in mammalian replication and evolution, FSH prepares the reproductive system for fertilization, implantation, pregnancy, and controls folliculogenesis, the oocyte is chosen, steroid hormone produce in the ovary [9]. In the male, it intercede testis evolution and spermatogenesis [10]. Luteinizing hormone (LH) is a heterodimeric glycoprotein that consists of a well-preserved alpha chain and a discrete beta subunit that give the biological specificity to the hormone-receptor interaction in target tissues. LH is produced by the anterior pituitary gland [11]. Testosterone is secreted by the testis and to a lesser extent by the adrenal glands, which is the main male sex hormone. The function of this hormone is the maturation of the male sexual organ, responsible for the secondary sexual characteristics [12, 13], the high levels of gonadotropins and testosterone cause the sperm to start forming and it lasts for life, in the old age, it decreases, and to produce mature spermatozoa, it requires 65-70 days from the first stage [14, 15].
Materials and methods
Experimental animals
The Animal House of the Biological Science Department at the College of Sciences/Misan University provided the male mice that were used in the experiment. The male mice were examined and were free of pathogens, with ages 8-12 weeks, weight 28 g, and type BABL/c, and then they were left for 2 weeks for adaptation.
Groups
The animals were divided into three groups: group 1 (control): 30 mice were used in the study, and they received normal saline orally treatment for six weeks. Amitriptyline was given orally to 30 mice in group 2 for six weeks, while escitalopram was given orally to 30 mice in group 3 for the same period. Antidepressants were administered at a dose (0.4 µl/day) twice a day.
Sample collection
According to the protocols endorsed by the regional animal ethics boards, the male mice were handled, and at the end of the second, the fourth, and the sixth weeks, by using euthanasia, the blood was taken from the heart according to [16]. By using a syringe (3 mL) and the blood was placed in tubes containing a substance that helps coagulation to obtain the serum measure the hormone levels (FSH, LH, and testosterone) by using a device (ELISA), and then the epididymis was taken to examine sperm parameter. According to [17], the sperm concentration was examined, and sperm motility was examined [18]. The dead sperm were examined [19].
Statistical analysis
The one-way ANOVA (Analyzes Variation) and an LSD test for the statistical differences were performed on the data's mean and standard deviation by using the SPSS program [20].
Results and Discussion
Sperm concentration
The findings of this study indicated that the sperms concentration decreased significantly (p< 0.05) in both the escitalopram and amitriptyline groups during the second week. However, the escitalopram group experienced a smaller decrease in sperm concentration than the amitriptyline group did as compared with the control group, which had sperm concentrations of (290.0±56.01), amitriptyline as (165.00±18.37), and escitalopram as (197.00±35.10).
The sperms concentration in the amitriptyline and escitalopram groups significantly decreased between the fourth and sixth weeks (p< 0.05), with the escitalopram group experiencing a smaller decline than the amitriptyline group, which had a significantly a higher concentration of sperms in the fourth week (282.00±22.80), amitriptyline was (158.00±25.88), and escitalopram was (161.80±44,40). In addition, the sperm concentration of the control group was (288.40±57.53) at the end of the sixth week, amitriptyline was (157.00±80.28), and escitalopram (158.00±39.46), as presented in Table 1.
Sperm motility
The findings of this study demonstrated that amitriptyline and escitalopram significantly reduced the sperms motility over six weeks, with escitalopram experiencing a smaller decrease than amitriptyline, in the second week, the control group's sperm motility was (81.80±4.49), whereas amitriptyline was (45.80±4.49) and escitalopram was (63.00±10.70), while the control group's sperm motility in the fourth week was (80.00±7.31), amitriptyline's in the fourth week was (41.00±7.03), and escitalopram's in the fourth week was (61.40±13.84), the control group's sperm motility in the sixth week was (82.20±5.40), amitriptyline's in the sixth week was (29.60±8.44), and escitalopram (54.00±6.55), as presented in Table 1.
Sperm dead
The findings of this study indicated that the number of dead sperms increased significantly (p<0.05) in both the amitriptyline and escitalopram groups compared with the control group during the second week, with the amitriptyline group experiencing a smaller increase than the latter, the control group's dead sperm count was (18.20±4.49), amitriptyline's was (34.20±11.45), and escitalopram's was (35.60±4.03).
As compared with the control group, the count of dead sperms in the amitriptyline and escitalopram groups showed a significant increase in the fourth week (p< 0.05); the increase in the escitalopram group was less than the increase in the amitriptyline group. The control group had a dead sperm count of (19.80±4.76), amitriptyline had a count of (38.60±8.73), and escitalopram had (33.80±7.15).
There was no significant difference (p>0.05) in the count of dead sperms in the amitriptyline group compared with the control group during the sixth week; the control group's dead sperm count was (18.80±2.16), while amitriptyline's was (25.80±8.04). However, the escitalopram group showed a significant increase (p<0.05) in the count of dead sperms compared with the control group; the escitalopram group with a dead sperm count was (33.80±13.16), as indicated in Table 1.
The result of our study is compatible with another study conducted [6], which demonstrated the use of antidepressant lead to the oxidative stress and production of reactive oxygen species (ROS) in the abundance and this is due to the damage the antidepressant dose to the membrane mitochondria. Numerous studies have shown that oxidative stress damages sperm through various aspects, including induction of DNA damage, lipid peroxidation of the plasma membrane of sperm, morphology, and impaired sperm motility [21]. The male mouse sperm concentration, motility, and death over a six-week period are depicted in Table 1.
Table 1: Shows the changes in the concentration, motility and dead of sperms of male mice over the six weeks

The values show the mean and standard deviation. A difference in small letters between groups indicates a statistically significant difference (p< 0.05). The similar little letters do not really mean anything different.
LH serum levels
The findings of this study revealed that the LH serum levels in both the amitriptyline and escitalopram groups decreased significantly (p< 0.05) over the course of the second and the fourth weeks. However, the loss of LH serum levels of the escitalopram group was less pronounced than that of loss of LH serum levels of the amitriptyline group, as compared with the control group, whose LH serum level in the second week was (0.01±0.00), amitriptyline was (0.002±0.00), and escitalopram was (0.004±0.00), the LH serum level of control group was (0.01±0.00), amitriptyline was (0.002±0.00), and escitalopram was (0.003 ±0.00) in the fourth week.
The LH serum level in the amitriptyline group was decreased significantly (p<0.05) over the course of the sixth week period, as compared with the control group, where it was (0.01±0.00), amitriptyline was (0.001±0.00). In contrast, there were no significant differences (p>0.05) in the LH serum level between the escitalopram group and the control group, where it was (0.04±0.06), as indicated in Table 2.
FSH serum level
The findings of this study indicated that the FSH serum level was significantly decreased (p<0.05) in the amitriptyline group over the course of six weeks, whereas there was no significant difference (p>0.05) between the escitalopram group and the control group over the same period. The FSH serum level of the control group in the second week was (0.01±0.00, amitriptyline was (0.001±0.00), and escitalopram was (0.01±0.00).
Whereas, the FSH serum level of the control group was (0.01±0.00) in the fourth week, amitriptyline was (0.001±0.00), and escitalopram was (0.01±0.00), in the sixth week, respectively. The FSH serum level of the control group was (0.01±0.00), amitriptyline was (0.001±0.00), and escitalopram was (0.03±0.06), as presented in Table 2.
Testosterone serum level
The findings of this study revealed that the testosterone serum levels in the amitriptyline and escitalopram groups were decreased significantly (p <0.05) over the course of the second, the fourth, and the sixth weeks. However, the decline of the escitalopram group was less pronounced than that of the amitriptyline group's, relative to the control group, whose testosterone serum levels in the second week were (9.48±0.85), amitriptyline was (0.41±0.06), and escitalopram was (2.21±0.05).
In contrast, the testosterone serum level of control group in the fourth week was (9.48±0.93), while its amitriptyline and escitalopram levels were each (0.12±0.07) and (2.56±2.23), respectively. In the sixth week, the testosterone serum level of control group was (9.48±0.83), amitriptyline was (0.74±0.15), and escitalopram was (3.54±1.48), as listed in Table 2 which disrupted the synthesis, release processes, transfer of hormones, and metabolism by drugs [22, 23] where many studies have shown the contact of the (HPG) axis. It is the regular neuronal and hormonal signals at different levels of the axis (HPG) that control neuroendocrine and reproductive functions [24]. The decreased FSH, and LH due to neuroendocrine factors such as serotonin causes increased prolactin hormone by inhibiting dopamine and acting to increase prolactin to suppress GnRH and its decrease causes a decrease in FSH and LH [25]. Increased serotonin causes dopamine inhibition and dopamine has an important role in the capacitation vitality and movement of sperm ability [26].
According to Table 2, the male mice's serum levels of LH, FSH, and testosterone changed throughout the course of six weeks.
Table 2: Shows the changes in the LH, FSH and testosterone serum levels of male mice over the six weeks

The values show the mean and standard deviation. A difference in small letters between groups indicates a statistically significant difference (p< 0.05). The similar little letters do not really mean anything different.
Histochemistry Study
The testis
The testis' basement membrane's contact with PAS was weak, according to the findings of the study in the control group's second week of testis. As displayed in Figure 1, the interaction was moderate in the testis sections of the amitriptyline and escitalopram group (see Figures 2 and 3).
According to the testis for the control group, the testis basement membrane's interaction with PAS was weak as of the fourth week. Figure 4 shows a moderate interaction between amitriptyline and escitalopram in testis sections from that group (see Figures 5 and 6).
A weak contact between the testis' basement membrane and PAS was visible in the control group's testis at the end of the sixth week. Amitriptyline and escitalopram revealed a substantial interaction in the testis sections in Figure 7, 8 and 9.
The epididymis
The epididymis for the control group in the study's second week revealed that there was only a moderate interaction between the basement membrane of epididymis and PAS. However, Figures 10, 11, and 12 indicate the interaction that was only mild in the epididymis regions for the amitriptyline and escitalopram groups.
The fourth week revealed a moderate interaction between the basement membrane and PAS in the epididymis of the control group, as depicted in Figure 13. The epididymis sections for the group receiving amitriptyline and escitalopram demonstrate a strong interaction, as exhibited in Figures 14 and 15.
A moderate interaction between the basement membrane and PAS was visible in the epididymis of control group at the end of the sixth week.
Table 3: Reaction epithelium with PAS in testis, epididymis


Figure 1: Two-week examination of the testes of control male mice shows (1) basement membrane reaction with PAS weak

Figure 2: Two-week examination of the testes of amitriptyline male mice shows (1) basement membrane reaction with PAS moderate

Figure 3: Two-week examination of the testes of escitalopram male mice shows (1) basement membrane reaction with PAS moderate (400x)

Figure 4: Four-week examination of the testes of control male mice shows (1) basement membrane reaction with PAS weak

Figure 5: Four-week examination of the testes of amitriptyline male mice shows (1) basement membrane reaction with PAS moderate

Figure 6: Four-week examination of the testes of escitalopram male mice shows (1) basement membrane reaction with PAS moderate

Figure 7: Six-week examination of the testes of control male mice shows (1) basement membrane reaction with PAS weak

Figure 8: Six-week examination of the testes of amitriptyline male mice shows (1) basement membrane reaction with PAS strong

Figure 9: Six-week examination of the testes of escitalopram male mice shows (1) basement membrane reaction with PAS strong

Figure 10: Two-week examination of the epididymis of control male mice shows (1) basement membrane reaction with PAS moderate

Figure 11: Two-week examination of the epididymis of amitriptyline male mice shows (1) basement membrane reaction with PAS moderate

Figure 12: Two-week examination of the epididymis of escitalopram male mice shows (1) basement membrane reaction with PAS moderate

Figure 13: Four-week examination of the epididymis of control male mice shows (1) basement membrane reaction with PAS moderate

Figure 14: Four -week examination of the epididymis of aitriptyline male mice shows (1) basement membrane reaction with PAS strong

Figure 15: Four-week examination of the epididymis of escitalopram male mice shows (1) basement membrane reaction with PAS strong

Figure 16: six-week examination of the epididymis of control male mice shows (1) basement membrane reaction with PAS moderate

Figure 17: Six-week examination of the epididymis of amitriptyline male mice shows (1) basement membrane reaction with PAS strong
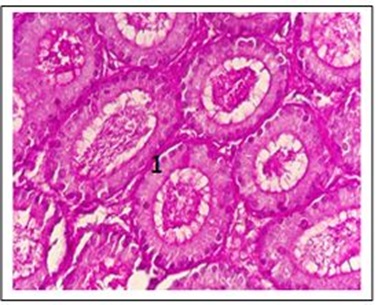
Figure 18: Six-week examination of the epididymis of escitalopram male mice shows (1) basement membrane reaction with PAS strong (400x)
As shown in Figure 16, there was a significant interaction between amitriptyline and escitalopram in the epididymis sections (see also Figures 17 and 18).
Our study results are in agreement with [27] who explained the reason for the PAS interaction with basement membranes is the oxidative stress caused by antidepressants.
The formation of hydroxyl molecules, the highly reactive oxidizing molecules, caused the fats oxidation, and thus caused damage to nucleic acid and proteins [28].
This study showed that both amitriptyline and escitalopram cause a decrease in the number and motility of sperms and an increase in the number of dead sperms as well as affecting hormones (FSH, LH, and testosterone), causing a decrease in the spermatogenesis process.
Conclusion
Our study showed that both Amitriptyline and Escitalopram cause a decrease in the number and motility of sperms and an increase in the number of dead sperms as well as affecting hormones (FSH, LH and Testosterone), causing a decrease in the process of spermatogenesis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
The author declared that they have no conflict of interest.
HOW TO CITE THIS ARTICLE
Israa Abdulameer Naeem, Ali Ali Khalaf. Hormonal, Histological, and Sperm Parameters: A Comparative Study between Amitriptyline and Escitalopram in Male Mice. J. Med. Chem. Sci., 2023, 6(3) 592-605


