Document Type : Original Article
Author
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Near East University, Nicosia, Cyprus
Abstract
To create chemical structures with various pharmacological actions, heterocyclic compounds play a key role as pharmacophores. The pharmacological effects of derivatives of 2(3H)-benzoxazolone include analgesic, anti-inflammatory, antibacterial, anti-nociceptive, and anticancer activities. The aim of this study was to examine the cytotoxic and apoptotic effects of newly synthesized 2(3H)-benzoxazolone derivatives against metastatic MDA-MB-231 breast cancer cell lines in vitro and in silico. The structural verifications of target compounds were performed by elemental analysis, FT-IR, and NMR spectra. MTT assay was used to assess the cytotoxic effects of the compounds in terms of the decreased cell viability. TUNEL assay was used to confirm the apoptotic activities of the compounds. The MTT results revealed that compound 2, which has a chlorine substituent at the 5th position of the core structure, had the maximum activity at a concentration of 50 µM during a 72-hour incubation period. The results demonstrated that 2(3H)-benzoxazolone derivatives with piperidine substituents efficiently reduced the cell survival in the target cell line. The molecular docking results also supported the experimental data. Furthermore, the in-silico investigation demonstrated that the synthesized compounds have desirable drug-like characteristics for the oral drug-delivery system. In addition, the findings showed that chlorination of the benzoxazolone ring influences the apoptotic activity, suggesting that these derivatives might be promising innovative anticancer medications in the future.
Graphical Abstract
Keywords
Main Subjects
Introduction
Cancer is the second largest cause of mortality worldwide and a potentially fatal condition [1]. Nearly 10 million cancer-related deaths were recorded by the World Health Organization (WHO) in 2020 [1]. The most frequent malignancy in women is breast cancer [2]. Anticancer research is crucial for the development of novel lead compounds, particularly in women because of the rise in breast cancer cases globally. Breast cancer research and novel drug synthesis models are being reported with an increase in breast incidence and mortality [3-9]. Given that there are several active sites on its basic structure, the 2(3H)-benzoxazolone scaffold plays a significant role in medicinal chemistry. The pharmacological effects of 2(3H)-benzoxazolone derivatives include analgesic [10], anti-inflammatory [10], antiviral [11], antifungal [12], anti-nociceptive [13], anticancer [14], and anticonvulsant [15] effects. Nevertheless, there are very few studies available that examine the cytotoxic and apoptotic effects of compounds based on benzoxazolone. Bilginer et al. have investigated 6-(3-aryl-2-propenoyl)-2(3H)-benzoxazolone derivatives for their antiproliferative efficacy against the colon cancer cell line, HCT116 [16]. In the same study, caspase-3 activation and DNA measurements were utilized to assess apoptosis in the treated cells, while lactate dehydrogenase (LDH) activity was evaluated to analyse necrosis [16]. In another study, similar chalcone-like benzoxazolone derivatives were synthesized, examined for cytotoxicity against the different human leukemia cell lines, and were found to be effective [17].
Disubstituted piperidines were also reported to demonstrate various biological activities in the literature [18]. In recent years, there have been several publications on studies evaluating the cytotoxicity of piperidine derivatives [19, 20]. Due to their high reactivity, Mannich bases, including piperidine derivatives, are crucial in the discovery of synthetic pathways in pharmaceutical chemistry [21]. Ognyan et al. investigated the potential anticancer properties of benzoxazolone Mannich bases containing trimethoxyphenyl propenoyl groups at positions 6 in the primary structure [22]. This study examined the target compounds for their cytotoxicity and discovered mild to moderate activity against a number of leukemia and breast cancer cell lines, including SKW-3, MDA-MB-231, HL-60, and BV-173. BV-173 had the highest chemosensitivity to the investigated compounds, followed by SKW-3 and HL-60 cell lines [22].
Apoptosis has been defined as programmed cell death or cell suicide. Apoptosis is an energy dependent and physiological event [23]. Numerous illnesses and diseases can be brought on by the apoptotic program being dysfunctional or out of balance [23]. In cancer cells, it is a targeted process. One of the most important and well-known indicators of apoptosis is caspase-3 [24]. It is a member of the cysteine-aspartate protease family, one of the six protease families with significant roles in both neuropathology and normal neuronal development. Caspases-3 eventually causes apoptosis, which involves DNA strand damage and cleavage, for both mechanistic routes [24]. Caspase-3 activity suggests that the downstream portion of apoptotic pathway has been reached. Therefore, caspase-3 is an important target in cancer research. Consequently, regardless of the intrinsic or extrinsic mechanism, apoptosis is a desired state in cancer cells. Among breast cancer cell lines, MDA-MB-231 is a triple-negative breast cancer (TNBC) cell line that is extremely aggressive, invasive, and poorly differentiated because it lacks the expression of the oestrogen receptor (ER), the progesterone receptor (PR), and HER2 (human epidermal growth factor receptor 2) [25]. MDA-MB-231 is one of the most commonly used cell lines in breast cancer research. The TUNEL assay may be used to analyse the DNA fragmentation during apoptosis by using fluorescence microscopy or flow cytometry techniques [26, 27]. Furthermore, the quantitative real-time polymerase chain reaction approach may detect the quantity of growth arrest-specific 2 (GAS2) in tissues [28].
All of these prior findings and a comprehensive literature review showed that compounds with benzoxazolone and piperidine scaffolds have considerable cytotoxic effects on several cancer cell types including MDA-MB-231. Further investigations incorporating cytotoxicity assessments of piperidine containing benzoxazolone derivatives indicate the potential in the creation of effective chemotherapeutic drugs. Based on these previous findings in the literature, the current work was launched with the goal of screening synthesized derivatives for the cytotoxic and apoptotic effects of benzoxazolone derivatives against MDA-MB-231 cell line. The experimental data were further supported by in silico molecular docking and ADME analysis for drug likeness of synthesized compounds. In silico molecular docking and ADME study for drug likeness of synthesized compounds supported the experimental findings.
Materials and methods
All chemicals were purchased from Merck (formerly Sigma-Aldrich, Darmstadt, Germany). Mettler Toledo FP900 Thermosystem (Mettler-Toledo, Greifensee, Switzerland) was used to measure the melting points and the data were uncorrected. FT-IR spectra were recorded as films on a Spectrum Two FT-IR Spectrometer (PerkinElmer, Inc., Waltham, MA, USA). The NMR spectra of substances synthesized in this work were obtained by using a Jeol 400 MHz spectrometer (Peabody, MA, USA). The chemical shifts were recorded in parts per million (ppm) units and tetramethylsilane (TMS) was used an an internal reference. The splitting patterns are denoted by the letters s (singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). Elemental analyses were examined for C, H, and N on Leco CHNS 932 analyser (Leco-932, St. Joseph, MI, USA) and the analyses were within ± 0.4% of the theoretical values.
General synthesis method
Mannich reaction was used for the synthesis, according to a previously described method [29]. 20 mmol 2(3H)-benzoxazolone derivatives and 20 mmol of 4-methylpiperidine were dissolved separately in the appropriate amount of methanol and mixed. Following that, 20 mmol of 37% w/v formaldehyde solution was added. The mixture was heated under reflux for 2 hours. After dumping the liquid into the ice bath, the significant precipitation occurred with a light yellow colour. The precipitate was filtered, rinsed with cold methanol, vacuum dried, and recrystallized in ethanol.
3-{[4-methylpiperidin-1-yl]methyl}-2-benzoxazolone (Compound 1)
Yellow powder, Yield: 81.50%; mp 92-94 °C, IR (KBr) (νmax/ cm-1): 2759-2949 (C-H), 1760 (lactam, C=O) cm-1; 1H-NMR (400 MHz, CDCl3): d 7.1-6.7 (m, 4H; Aromatic), 4.6 (s, 2H, methylene), 3.1 and 1.6 (dd, 4H, piperidine H6-H8), 2.3 (t, 4H, piperidine H5-H9), 1.2-1.4 (m, 1H, piperidine H7), 0.8 (d, 3H, H10), 13C-NMR (100 MHz, CDCl3): δ 155.4, 143.3, 131.2, 124.6, 121.5, 108.7 (Aromatic-C), 65.2 (methylene-CH2), 51.2, 34.0, 30.1 (piperidine-C), 21.7 (methyl-CH3). Anal. Calc. for C14H18N2O2 C, 68.27; H, 7.37; N, 11.37; Found C, 68.24; H, 7.34; N, 11.35.
5-chloro-3-{[4-methylpiperidin-1-yl]methyl}-2-benzoxazolone (Compound 2)
Yellow powder, Yield: 74.5%; mp 108-110 °C; IR (KBr) (νmax/ cm-1): 2759-2949 (C-H), 1760 (lactam, C=O) cm-1; 1H NMR (400 MHz, CDCl3): d 7.1-7.3 (m, 3H, Aromatic), 4.6 (s, 2H, H4), 3.1 and 1.6 (dd, 4H, piperidine H6-H8), 2.3 (t, 4H, piperidine H5-H9), 1.2-1.4 (m, 1H, piperidine H7), 0.8 (d, 3H, H10). 13C-NMR (100 MHz, CDCl3): δ 158.5, 143.3, 131.2, 124.6, 121.5, 108.7 (Aromatic-C), 65.2 (methylene-CH2), 51.2, 34.0, 30.1 (piperidine-C), 21.7 (methyl-CH3). Anal. Calc. for C14H17ClN2O2 C, 59.89; H, 6.10; N, 9.98; Found C, 59.81; H, 6.08; N, 9.96.
Cell culture and MTT assay procedure
Dulbecco’s modified eagle medium (DMEM) (Gibco) was used to grow the human breast cancer cell lines MDA-MB-231 (HTB-26, ATCC), which were supplemented with 10% fetal bovine serum (FBS), human insulin of 4 mg/mL, and 1% penicillin/streptomycin. The cells were kept at a constant temperature of 37 °C in a humidified incubator with 5% CO2. By using the MTT assay, the reduction in cell viability was examined. All stock solutions of compounds were dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) and diluted in growth media at various concentrations. In 96-well plates (2 × 103 cells/well), cells were plated. Cells were serum deprived for 24 hours in culture media with 0.1% FBS after they had reached around 80% confluence. 500 ng/mL to 2.5 g/mL of RANKL (Sigma-Aldrich) was used to stimulate cells for 24 hours. The media was changed for 200 µl of new medium, and each well received 50 µl of MTT reagent (5 mg/mL; Sigma-Aldrich). After 4 hours incubation period at 37 °C, the medium was changed for 200 µl of DMSO and 25 µl of glycine buffer. An ELISA reader (Labsystem Multiskan Ms) operating at 570 nm was used to measure the absorbance. Untreated cells were assumed to have a 100% absorbance rate. The studies and chemical dilutions were carried out three times.
TUNEL assay and apoptosis
By using a commercial in situ apoptosis detection kit (In Situ Cell Death Detection Kit AP, Roche), DNA fragmentation was discovered by labelling the apoptotic cells with a particular staining. The DNA fragmentation results from the activation of Ca/Mg-dependent endonuclease enzymes in apoptotic cells. On 6-well plates with coverslips, 5 × 104 MDA-MB-231 cells were cultured for 24 hours. The cells were fixed in a 4% paraformaldehyde solution after being rinsed with PBS. To improve permeability, the cells were treated in 0.1% Triton X-100 in 0.1% sodium citrate for 1 hour at 4 °C. The cells were then exposed to compound 1 and compound 2, respectively for 1 hour at 37 °C in the dark. Cells were further exposed to the medium alone. A light microscope was used for analysis (Olympus BX40, Tokyo, Japan).
Molecular docking and ADME analysis
The synthesized compounds and X-ray crystal structure of human caspase-3 (PDB ID: 5I9B) in complex with PRD_000238, native ligand [30-32] were prepared by using LigPrep and Protein Preparation Wizard in Maestro of Schrödinger-2021 software package, respectively [33-35]. The resolution of the crystal structure of human caspase-3 was determined as 1.80 Å. The standard precision module of the Schrödinger Suite (Glide SP) was utilized to perform the molecular docking calculations [36]. The native ligand’s docking pose and crystal conformation were discovered to be 1.291 Å. The 2D interactions were investigated by using Discovery Studio Visualizer software. The pharmacokinetic characteristics and drug-likeness of the synthesized compounds were assessed by using the preADMET prediction service depending on the 2D molecular structure of the studied derivatives.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). GraphPad Prism 9 software was used to evaluate the results. Where relevant, differences between groups were analysed statistically by using the Mann-Whitney test, with a p-value <0.05 indicating statistical significance.
Results and Discussion
Synthesis and molecular docking
Through the Mannich reaction, benzoxazolone derivatives, and 4-methylpiperidine were used to create Mannich bases, as displayed in Figure 1. Condensation in the formaldehyde presence resulted in the formation of a methylene bridge between these two structures. Indicating that the Mannich reaction was successful, the synthesized compounds had IR stretching bands of the lactam ring at 1760 cm-1 (C=O) and lacked the N-H bands of the piperidine and benzoxazolone rings, which typically are presented between 3100 and 3400 cm-1. At 4.6 ppm, a singlet signal was detected, demonstrating the presence of a methylene bridge. In addition, the aromatic protons in 1H-NMR spectra are visible as a multiplet between 6.7 and 7.3 ppm. The integral values match to the proposed structures of the synthesized compounds.

Figure 1: General synthesis method of compounds 1 and 2
Furthermore, a docking study was performed on synthesized compounds against caspase-3 enzyme. The docking scores and their common interacting amino acids with the active site of caspase-3 are listed in Table 1 for each derivative and the native ligand (PRD_000238). The 2D ligand-receptor interactions and electrostatic investigation of compound 1 and compound 2 in the active site of caspase-3 are depicted in Figures 2 and 3, respectively. Compared the synthesized compounds with the native ligand, the two compounds had similar docking scores and shared interaction residues. Moreover, both compounds produced an H-bond with THR77 and TYR276, which is thought to be one of the essential amino acids for caspase-3 activation. On the other hand, a hydrophobic pocket including the amino acid residues LEU81, LEU223, and LYS224 was created when a chlorine substituent was presented at position 5 of the benzoxazolone ring. This hydrophobic pocket appears to affect docking scores, implying that in future studies, any additional hydrophobic groups, such as small-chain alkyl groups like methyl or ethyl substituents could be replaced with chlorine at position 5 and tested for their effects on how similar derivatives interact with caspase-3.
Table 1: The docking scores of the synthesized derivatives and their common amino acid interactions with the native ligand in the binding pocket of caspase-3
|
Compound’s Name |
Docking Score (kcal/mol) |
Interacting Amino Acids |
|
Native Ligand (PRD_000238) |
-8.582 |
THR77, ARG207, SER209, LEU223, LYS224, TYR276. |
|
Compound 1 |
-7.245 |
THR77, LEU223, LYS224, TYR276 |
|
Compound 2 |
-7.868 |
THR77, LEU223, LYS224, TYR276 |

Figure 2: The 2D ligand-receptor interactions (on the left) and electrostatic investigation (on the right) of compound 1 in the caspase-3 active site

Figure 3: The 2D ligand-receptor interactions (on the left) and electrostatic investigation (on the right) of compound 2 in the caspase-3 active site
ADME properties and drug-likeness assessment
The analysis of the ADME characteristics of bioactive compounds is a key restriction throughout drug development that influences compound selection [37]. Around half of the applicants failed throughout the early stages of development due to unsatisfactory ADME profiles [38]. To avoid this failure, in-silico approaches for predicting pharmacokinetic features of the bioactive compounds and guiding the early phases of drug development have been efficiently employed. Furthermore, investigating pharmacokinetic features using in silico techniques prior to do the experimental studies assists in the identification of potential lead molecules. The evaluation of ADME properties were summarized in Table 2. The synthesized compounds initially displayed a moderate Caco-2 cell line permeability and an outstanding human intestinal absorption (HIA) value of 99%.
These results suggest that the other processes besides a passive diffusion may be involved in the absorption via the gut. This is due to the lack of carrier proteins, cells responsible for the secretion of mucus, and extracellular elements, which can similarly affect absorption in Caco-2 cell models [39]. In addition, the Caco-2 system's tight connections reduce the amount of substances that may pass through to be absorbed paracellularly [39]. Furthermore, the compounds were investigated to block the CYP2C9 enzyme, which might result in drug interactions with medications depending on CYP2C9 for their metabolism. Since just the free portion of the drug is responsible for pharmacological effect [40], the high plasma protein binding capacity might impact the drugs’ efficacy. Both compounds' plasma protein binding capacities were not particularly high. Therefore, they could exhibit the optimal plasma half-life, distribution volume, and clearance rates. Moreover, the synthesized compounds were impermeable across blood-brain barrier. When an attempt to prevent or address the brain functions throughout the drug design and synthesis stage, brain penetration is a crucial issue to take into account. The danger of harmful CNS side effects and toxicity is decreased or eliminated when compounds are not permeable to the blood-brain barrier [41].
Besides, Lipinski's rule of five consists of four physicochemical parameter criteria [42], which include the H-bond donors and acceptors, molecular mass, and logP value that define the drug-likeness for the oral administration system. In general, oral medications need these criteria to exhibit excellent intestinal permeability and water solubility profiles. Poor bioavailability and absorption are probably going to occur. The in-silico study revealed that all synthesized compounds followed Lipinski's rule. Thus, these compounds have a greater chance of commercial success due to the decreased attrition rates throughout drug design and clinical investigations.
Cell viability
Target compounds were administered to MDA-MB-231 cells at several doses (5, 10, 15, 20, and 50 μM) for 48 and 72 hours, respectively. The acquired data demonstrated a dosage and time-dependent substantial reduction in cell viability for all doses of both compounds compared with the control group. The MTT results of compound 1 are demonstrated in Figure 4. According to the results, compound 2 with a chlorine substituent in place of hydrogen at position 5 of the primary structure was generally more effective at lowering the percentage of cell viability. In addition, both compounds were most efficient in inhibiting cell growth for a 72-hour incubation period at 50 μM concentrations.
cells
The MDA-MB-231 cells that had been treated with 50 μM doses of both compounds for 72 hours, the apoptotic effects of the investigated compounds were assessed by using the TUNEL assay. The findings show that both compounds are efficient in preventing DNA fragmentation in MDA-MB-231 cells during apoptosis. The results are summarized in Table 3. TUNEL analysis of DNA fragmentation in MDA-MB-231 cells treated with synthesized compounds under light microscope is indicated in Figure 5.
Table 2: Some important in silico based pharmacokinetic properties of synthesized compounds
|
Compound |
Lipinski’s rule of five |
BBB |
HIA% |
PPB % |
Caco2-P (nm/sec) |
CYP2C9 blockage |
|
Compound 1 |
Fit |
0.21 |
99.39 |
47.18 |
40.43 |
Nil |
|
Compound 2 |
Fit |
0.44 |
99.09 |
64.61 |
51.80 |
Nil |
BBB: Blood-brain barrier penetration of the compound, HIA: Human intestinal absorption, PPB: Plasma protein binding ability, Caco2-P: Caco2 (human colorectal carcinoma) cell permeability, and CYP2C9: Cytochrome-P450 2C9

Figure 4: The effects of compound 1 and compound 2 on cell viability of MDA-MB-231 cells
Table 3: The results were reported as means ± SD and were significant compared with the control group for compound 1 (p< 0.01) and compound 2 (p< 0.001). Data were compared by using the Mann-Whitney test
|
Compound 1 |
Compound 2 |
Control Group |
|
71.63 ± 10.98 |
83 ± 6 |
34.84 ± 4.02 |
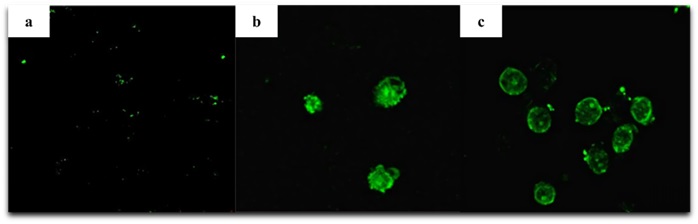
Figure 5: Evaluation of DNA fragmentation by TUNEL assay in MDA-MB-231 cells exposed to synthesized compounds. a) Cells without treatment b) Cells treated with 50 μM of compound 1 for 72 hours c) Cells treated with 50 μM of compound 2 for 72 hours
Conclusion
The cytotoxic and proapoptotic characteristics of these newly synthesized 2(3H)-benzoxazolone derivatives against MDA-MB-231 cell line were screened for the first time. Inhibiting the growth, proliferation, and induction of apoptosis in these metastatic breast cancer cells was more effectively accomplished by compound 1 than by compound 2. Furthermore, a TUNEL assay investigation was used to confirm these findings. The TUNEL assay data revealed that both compounds were capable of inducing DNA fragmentation during apoptosis in MDA-MB-231 cells. These results clearly demonstrated that the substitution of 5th position of the main ring affected the apoptotic process. The presence of a chlorine substituent (compound 2), on the other hand, altered docking scores in molecular docking analysis, providing a much more desired result. A comprehensive investigation of how different types of substituents affect all possible cancer pathways through connected derivatives of these substances would be valuable. Using immunohistochemical analyses, the additional evaluation of these drug candidates' possible apoptotic pathways could be carried out.
Acknowledgments
The author would like to thank the Near East University, Faculty of Pharmacy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
The author declared that they have no conflict of interest.
ORCID:
Emine Erdag
https://orcid.org/0000-0002-1431-935X
HOW TO CITE THIS ARTICLE
Emine Erdag. Evaluation of 2(3H)-benzoxazolone derivatives containing piperidine substituents as cytotoxic and apoptotic agents: An in vitro and in silico study. J. Med. Chem. Sci., 2023, 6(3) 569-579


