Document Type : Original Article
Authors
1 Al-Iraqia University, Medical College, Medical Microbiology Department, Baghdad, Iraq
2 Assistant professor Dr. Sanaa Khudhur Jameel Al-Iraqia University, Medical College, Medical Microbiology Department, Baghdad, Iraq
3 Rheumatology Unit, Department of Internal Medicine, Baghdad Teaching Hospital, Medical City Complex, Baghdad, Iraq
Abstract
Although the etiology of RA is complicated, infection with various microorganisms can activate several immune system pathways, potentially leading to an autoimmune response. Urinary tract infections were significantly more common in RA patients. Interleukin 18 is crucial in the pathogenesis of rheumatoid arthritis. A case-control study included 50 patients with rheumatoid arthritis indicating urinary tract infection symptoms, diagnosed by the rheumatologist according to the criteria of ACR/EULAR 2010, compared to 40 healthy subjects of the same age and sex who served as the control. For routine laboratory tests such as C-reactive protein(CRP), erythrocyte sedimentation rate (ESR), rheumatoid factor (RF), and anti-cyclic citrullinated peptide (anti-CCP), in addition to the estimation of serum concentration of IL18 by ELISA, to detect UTI, midstream urine samples were obtained aseptically, and samples were diagnosed using accepted techniques. The results of this study revealed that 44(88%)of patients with rheumatoid arthritis were found to have UTI, compared to the control group (88% vs. 17.5%), with an odd ratio (alternative for relative risk) of 34.571. In the investigation of the study groups, an equal proportion of gram-positive and gram-negative bacteria were identified among the case groups (each 50%). Among isolates, Escherichia coli (E.coli) was the predominant isolated bacteria (73%),and the gram-positive bacteria among the case groups were mainly Staphylococcus haemolyticus (41%). The mean values of IL18 compared to the control group were found to be considerably higher among the case group.
Graphical Abstract
Keywords
Main Subjects
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that causes stiffness, pain, and swelling in the joints [1]. The condition may cause deformity, disability, and joint destruction in addition to extra-articular complications. The small joints in the hands are most frequently affected by the initial inflammatory arthritic sign of rheumatoid arthritis [2]. Rheumatic disorders are regarded as a serious concern to public health globally, but the cause of the growing prevalence is especially significant in developing countries [3]. The clinical link between infection and RA has been established in several investigations. Infection is frequently discovered early in the RA course and can occur before clinical arthritis, implying that infection plays a role in the onset and exacerbation of the disease [4, 5]. A urinary tract infection (UTI) is defined as an infection of the lower or the upper genitourinary tract that is diagnosed based on the presence of a pathogen in the urinary tract and the symptoms that accompany it [6]. UTIs are believed to be the most prevalent infection worldwide, with a global prevalence ranging from 0.7% to 19.6% in rich countries. This is relevant in both hospital and community settings. The prevalence (i.e. 24%) is significantly higher in developing countries such as Iraq [7]. The gram-positive and gram-negative bacteria are linked to UTIs. The most common cause of UTIs is Escherichia coli. The Enterobacteriaceae family, includes Klebsiella, Proteus, Citrobacter, and Enterobacter spp., as well as Enterococcus species, Pseudomonas species, Streptococci, and Staphylococci are among the detected additional organisms [8]. Antimicrobials, which are used to prevent and manage bacterial infections, are probably one of the most successful forms of treatment in medical history. Antibiotics are crucial to preventing, combating, and eliminating bacterial resistance [9]. Fimbrial adhesions expressed by uropathogenic bacteria bind to glycolipids and glycoproteins on the epithelium surface. Bacteria can withstand the passage of urine into the urinary system in this way and persist there. In addition, bacteria create toxins, hemolysin, and colony necrotizing factors. These substances alter epithelial integrity, enable bacterial penetration, and raise infection risk as a result. In addition, uropathogens can incorporate into host epithelial cells and proliferate there, creating a reservoir for recurrent infection [10]. Both sexes and people of varying ages can develop UTIs. However, because of some factors including the female urethra's closeness to the anus and hormonal activity, females are more likely to develop UTIs than males [11].
In the pathophysiology of rheumatoid arthritis, cytokines play a significant role [12]. A major class of cytokines known as interleukins plays a key role in the triggering of immunological reactions such as inflammation. Once an IL has been created, it travels to its target cell and attaches to it through a receptor molecule on the cell surface. This interaction triggers off a series of signals inside the target cell that eventually change the cell's behavior [13]. Regulatory T cells mediate tolerance to self-antigens, whereas Th17 cells are involved in the pro-inflammatory response to infections. RA and other autoimmune diseases disturb the Treg-Th17 balance [14]. Interleukin IL-18 is strongly expressed in the serum, synovial tissue, and synovial fluid of patients with RA, and is associated with disease activity [15]. Interleukin IL-18 is a structurally identical pro-inflammatory cytokine to IL-1 and belongs to the IL-1 superfamily. Interferon-gamma (IFN-G) production is enhanced by IL-18, which triggers a strong Th1 response [16]. IL-18 may play a role in the progression of RA [17]. The severity of the disease appears to be correlated with IL-18 concentrations in the serum and affected joints. Without external stimulation, synovial cells in patients with RA create interleukin-18. It has the power to stimulate synoviocytes, prompting them to release inflammatory chemicals like TNF-gamma (TNF-G), IL-1, chemokines, and adhesion molecules that destroy cartilage. The pro-inflammatory cytokines interferon-gamma (IFN-G) is produced as a result of memory T cell activation in injured joints, which is also triggered by IL-18 [18].
Materials and methods
From November 2021 to March 2022, this case-control study was carried out in the Baghdad Teaching Hospital, Medical City, Rheumatology Consulting Clinic on 50 patients with rheumatoid arthritis (46 females and 4 males) showing symptoms of urinary tract infection diagnosed by the rheumatologist according to the ACR/EULAR 2010 criteria compared with 40 healthy subjects of the same age and sex served as control. The study was conducted after approval of Medical College of Al-Iraqia University and Iraqi Ministry of Health, and patients provided consent to collect urine samples, draw blood samples, and answer the questionnaire. The mean age of the cases group was 48.92 ± 10.700 years old mostly in the age group of 51-60 years old (36%), and that of controls was 46.0 ± 11.944 years old mostly in the age group of 51-60 years old (32.5%). The disease activity (CDAI) has been calculated for every patient.
5 mL of peripheral venous blood was aspirated from each patient and control subject. Regular laboratory tests for systemic work-up include measuring ESR by the Westergren method, and RF, CRP, anti-CCP antibodies, and serum IL-18 levels by using the ELISA technique with commercially available kits (MyBioSource-USA) by the manufacturer's recommendations.
To detect urinary tract infection, after first cleaning the genitalia, the urine samples were collected by using an aseptic technique in a 20 mL sterile screw-capped container that was distributed to the patients. To avoid contamination, midstream urine (MSU) was collected from patients with rheumatoid arthritis and healthy individuals (as a control). 10 ml urine sample was centrifuged for 10 minutes at 3000 rpm to inspect the deposit and underwent a microscopic examination before being cultured using a high- power objective lens (40x) for the leukocytes presence. All urine samples were inoculated on blood agar and MacConkey agar, incubated at 37 °C for 24 hours and 48 hours in negative cases, and then the bacterial growth, the presence of >105 identical colonial morphotypes (105 CFU/ml) and >5 pus cells per high-power field were considered morphological evidence of a UTI. Bacterial colonies were initially classified by gram stain morphology, and then definitively identified according to the standard culture and biochemical characteristics of isolates by using the Vitek-2 system (bioMerieux) according to the manufacturer,s instructions.
Statistical analysis
The data were entered, checked, and analyzed by using computer software programs of statistical package of social sciences (SPSS) version 26 and STATISTICA version 9. The descriptive statistics of frequency distribution tables, number and percentage for qualitative data, and mean, standard deviation and range for quantitative data were used. Unpaired t-test, One Way ANOVA test, and Chi-square test (alternative Likelihood ratio) were used to identify the significant differences between study groups of cases and controls regarding different quantitative and categorical parameters, respectively. A P-value of < 0.05 was used to determine statistical significance throughout the study.
Results and Discussion
Out of 50 rheumatoid arthritis patients, 44 (88%) were confirmed to have a UTI. It was discovered that the case group's UTI was much higher than that of the control group (88% vs. 17.5), with an odd ratio (alternative for relative risk) of 34.571 (95%CI: 10.622-112.524). The obtained results are depicted in Table 1. One of the most common routes by which infectious or chemical agents can cause autoimmunity is by molecular mimicry. It occurs when similarities between the foreign and self-peptides encourage the activation of auto-reactive T or B cells in a susceptible individual by a foreign-derived antigen [19].
Table 1: Distribution of urinary tract infection among study groups (n= 90)

Concerning the investigation of urine culture of study groups, an equal proportion of gram-positive and gram-negative bacteria were identified between the case groups (each 50%), while the gram-negative bacteria was more than gram-positive between the control groups (57% vs. 43%), respectively, as displayed in Figure 1.
Escherichia coli (E. coli) was the predominant isolated bacteria among the case group (73%), followed by Klebsiella pneumoniae (9%), Proteus mirabilis, Morganella morganii, Pseudomonas aeruginosa, and Acinetobacter baumannii (each 4.5%). However, only Escherichia coli (E. coli) was the isolated as gram-negative bacteria among the control group (100%), as illustrated in Figure 2. Pathogenesis is considered to originate with urethral colonization, and then bacteria ascend into the bladder, where they proliferate, and finally they pass into the urine. Following this, UPEC cells engage the bladder's epithelial defense mechanism by adhering to the bladder surface increasingly. Biofilms develop eventually. Following invasion (via bacterial replication and the development of intracellular bacterial communities), the UPEC cells may ascend to colonize the kidneys, where they may cause tissue damage and raise the risk of sepsis [20].
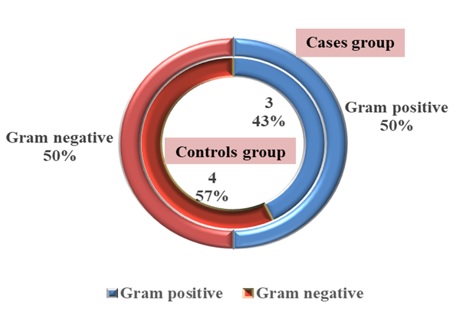
Figure 1: Distribution of urinary culture among study groups (n= 90)

Figure 2: Distribution of isolated Gram -negative bacteria among study groups (n= 90)
Moreover, the isolated gram-positive bacteria among the cases group were mainly Staphylococcus haemolyticus (41%) followed by Staphylococcus aureus, Staphylococcus hominis, Staphylococcus saprophyticus, and Enterococcus faecalis (27%, 14%, and 4%), respectively, whereas Staphylococcus aureus was the only gram-positive bacteria that had been isolated among the control group (100%), as demonstrated in Figure 3. Staphylococcus aureus is an opportunistic pathogen that infects both humans and animals with different types of diseases [21]. Lerbech et al. indicated that Staphylococcus haemolyticus is the most frequent pathogen (75%), which is consistent with our results [22].
Apart from the control group, the mean duration of rheumatoid arthritis was 8.42 ± 5.466 with most of the patients suffering from it < 12 years (80%). On the other hand, the level of disease activity among the case group was mainly moderate (70%) followed by low and high (20% and 10%), respectively. In the blood parameters among the study groups, it has been found that the mean level of the Erythrocyte Sedimentation Rate (ESR) was significantly higher between the case group than the controls (42.52 ± 21.205 vs. 12.18 ± 5.310), respectively with a significant difference of 30.345 (t= -8.823, df:88, P= 0.000). Similarly, the mean level of C- reactive protein (CRP) was substantially higher in the cases group compared to the controls (2.77484 ± 1.438752 vs. 1.17528 ± 0.502372), respectively with significant differences of 1.599565 (t= -6.706, df: 88, P= 0.000). Various metrics are utilized to measure the severity of rheumatoid arthritis (RA). Although there is currently no clear consensus on when to administer one, the other, or both, the laboratory tests like the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) have been a crucial component of doctors' tools for many years. To measure acute disease activity among these tests, CRP has emerged as the most widely used serological marker [23].
Likewise, the mean concentrations of anti-citrullinated protein/peptide antibody (ACPA) and rheumatoid factor (RF) were significantly higher among the cases group of rheumatoid arthritis than those of the rheumatoid arthritis free group (controls) (0.36290 ± 0.120678 vs. 0.23463 ± 0.029051) (t= -6.565, df: 88, P= 0.000), and (0.29276 ± 0.144911 vs. 0.19488 ± 0.25063) (t= -4.217, df:88, P= 0.000), respectively with significant differences of 0.128275 and 0.097885, respectively. The results are listed in Table 2. The two most notable autoantibodies in RA, rheumatoid factor and anti-citrullinated protein antibodies, offer various clinical and pathophysiology findings. They suggest a pathogenetic involvement in RA since they come before the start of disease symptoms and indicate a more severe disease course [24].

Figure 3: Distribution of isolated gram-positive bacteria among study groups (n= 90)
Table 2: Mean comparison of blood parameters among study groups (n=90)

When we compared the study’s groups regarding the immunological parameter of interleukin-18 significant differences were identified, as the mean value of interleukin-18 was observed to be considerably greater in the cases group compared with the healthy participants (329.62226 ± 57.864053 vs. 164.03333 ± 40.898986), respectively (t= -15.292, df: 88, P= 0.000) with significant differences of 165.588935. As represented in Table 3, IL18 plays a physiological role in inducing inflammatory disorders [25].
The results of this study showed that there were no significant variations among the means of interleukin-18 among patients with rheumatoid arthritis with duration of either less or more than 12 years, respectively (P> 0.05). Also, no detectable differences were recovered in the means of interleukin-18 among patients with rheumatoid arthritis with low, moderate, and high disease activity scores, respectively. The results are listed in Tables 4 and 5. Cardoso et al. demonstrated the IL18 level was significantly increased in RA patients compared with healthy donors, and no significant differences were found between CDAI and the level of IL18 in individuals suffering from rheumatoid arthritis [26]. To supply patients who do not respond to currently available treatments with new medications, it is essential to increase the understanding of the mediators involved in the RA pathogenesis and develop new targeted therapeutic [27].
Table 3: Mean comparison of immunological parameters among study’s groups (n=90)

Table 4: Mean comparison of immunological parameter based on the disease duration between the cases groups (n=50)
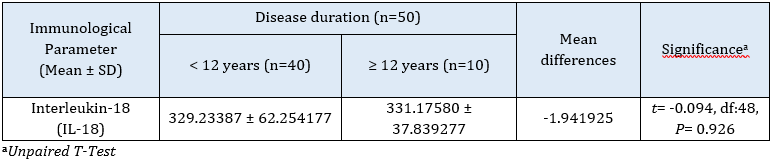
Table 5: Mean comparison of immunological parameters among cases groups’ disease duration (n=50)

Conclusion
UTI is one of the most microbial infections that have been linked to the development and progression of RA. Due to the function that IL18 plays in the pathophysiology of RA, a greater understanding of the complicated link between microbial pathogens and RA may one day assist to create efficient methods to prevent the early stages of the disease, avoiding the onset of the clinical phase.
Acknowledgments
The authors would like to thank all the participants who participated in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
There are no conflicts of interest in this study.
HOW TO CITE THIS ARTICLE
Esraa Mohammed Jasim, Sanaa Khudhur Jameel, Nabaa Ihsan Awadh. Evaluation of Urinary Tract Infection and Measurement of Interleukin 18 in Iraqi Patients with Rheumatoid Arthritis. J. Med. Chem. Sci., 2023, 6(2) 440-448
https://doi.org/10.26655/JMCHEMSCI.2023.2.24
URL: http://www.jmchemsci.com/article_156431.html


