Document Type : Original Article
Authors
Department of Chemistry, College of Science, University of Babylon, Babylon 51002, Iraq
Abstract
Three organotin complexes were successfully synthesized via condensation reactions of cephalexin) ligand (and di-organotin (IV) complexes in the presence of sodium hydroxide. Infrared, nuclear magnetic resonance (1H, 13C, 119Sn NMR) and elemental analyses were used to characterize the produced compounds. Based on the spectrum observations octahedral geometry was proposed for the complexes synthesized. This study looked at the antioxidant activity of tin (IV) compounds using two methods free radical scavenging activity (DPPH) and the CUPRAC method. Dioganotin (IV)-cephalexin complexes showed higher antioxidant activity than ligands due to the metal moiety, while complex (Me2SnL2) showed higher antioxidant activity than other complexes.
Graphical Abstract
Keywords
Introduction
Organotin compounds include at least one covalently direct bond between the organic group's carbon atom (C) and the tin (Sn) atom at the center. Organotin components are classed as mono, di, tri and tetra-organotin compounds (RSnX3, R2SnX2, R3SnX and R4Sn) where R represents an alkyl or aryl group and X represents any anionic species (carboxylate, hydroxide, halide, thiolate or oxide). The quantity of tin-carbon bonds as well as the length of the alkyl groups has a significant influence on the physical and chemical characteristics of organotin. Organotin (IV) compounds were identified to exhibit a broad variety of biological functions. Organotin (IV) carboxylate complexes have attracted special attention among several organotin (IV) compounds with biological molecules due to their greater biological activity than other organotin (IV) complexes with numerous ligands [1,2]. Several studies on organotin carboxylates have been carried out using functional carboxylic acids and donor groups O, N or S. Organotin carboxylates have a well-known structural variety. In the presence of additional coordinating atoms, the electronic and steric characteristics of organic alternatives on the tin and/or carboxylate moiety considerably influence the structural features of tin carboxylates. Some organotin carboxylates, such as hexameric cycle forms, have distinct structures [3-6]. Varieties of coordination geometries are present, as evidenced by the presence of monomeric, dimeric, tetrameric, oligomeric ladder, cyclic and drum structures. As a result, the synthesis of new organotin carboxylates with diverse structural properties will assist in the development of numerous medicinal and industrial applications such as anti-tuberculosis, anticancer drugs, antioxidants, plastic stabilizers, polymer catalysts, etc. [7-12]. Changes in the alkyl and aryl substituents of organotin (IV) have a major effect on the biological activity of these complexes. Based on their reactivity, employ organotin compounds in biological activities, which include antioxidants [13]. Antioxidants are substances that can either prevent or reduce the harmful effects of oxidants. These operate by donating one electron to an oxidant molecule, so decreasing its activity. Some researchers are looking for metal-based antioxidant medicinal mixes, particularly organotin (IV) complexes, to help minimize oxidative damage related to aging, skin diseases, cancer, inflammation, heart disease and malaria [14-17]. The antioxidant activity of various diorganotin (IV)-Cephalexin complexes was evaluated using diphenylpicrylhydrazyl (DPPH) and CUPRAC methods.
Materials and Methods
Chemicals and solvents utilized in this domain were of the best quality without any further filtering. Sigma-Aldrich (Schnelldorf, Germany) provided methanol, Cephalexin, diphenyl tin dichloride, dimethyl tin dichloride and dibutyltin dichloride.
Creation of Organotin (IV) Complexes
Di-organotin (IV) Complexes Synthesis [18]
The molar ratios (Metal: Ligand) to synthesize the complexes are 1:2, since a suitable amount of Ph2SnCl2 (1.3752 g, 4 mmol), Bu2SnCl2 (1.2153 g, 4 mmol) and Me2SnCl2 (0.8787 g, 4 mmol) were dissolved in 20 mL of methanol and then they were added to the stirred solution of cephalexin (2.7791 g, 8 mmol, in 30 mL methanol, in the presence of an appropriate amount of sodium hydroxide). This mixture was refluxed for 5 hours. The resulting solution was filtered, dried and recrystallized to form precipitation (Scheme 1).
The activity of antioxidant
Both the DPPH and CUPRAC procedures were used to test the antioxidant activity of the ligand and its complexes.
Diphenylpicrylhydrazyl (DPPH) Method
The ability of samples and standards-tannic acid to inhibit electrons was controlled by decolorizing a purple-colored DPPH methanol solution. A microplate reader was used to measure the absorption density at 490 nm. The samples were tested at different times by using a control (Methanol and DPPH solution), as shown in the following equation:
% Inhibition of the activity of DPPH = (A-B / A) ×100 (1)
Where, A = control absorb density and B = sample absorb density.
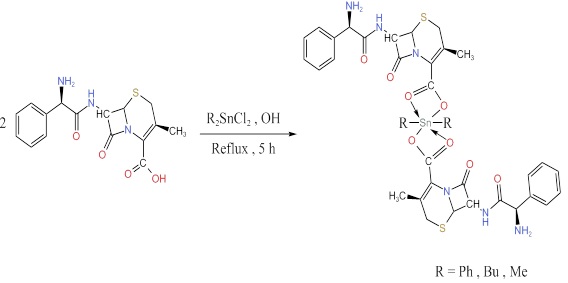
Scheme 1: Synthesis of Di-organotin (IV) complexes
CUPRAC Method
Standard solutions for the sample antioxidants were prepared with 1.0 x 10-4 M tannic acid [19], as demonstrated in the following equation:
Total antioxidants levels = (Atest / ASTD) × Conc. of STD (mmole/L) (2)
Results and Discussion
Physical data
The elemental composition of the diorganotin (IV)-cephalexin complexes was determined using elemental analysis. The results agree with the calculated values of the ligand (Cephalexin) and its complexes in general (Ph2SnL2, Bu2SnL2 and Me2SnL2). Table 1 shows the elemental analysis data of (C, H, N, S and Sn %) colors, melting points and yields of diorganotin (IV)-cephalexin complexes along the ligand (L).
Table 1: Physical data for complexes containing the ligand cephalexin.
|
Compounds |
Colors |
Melting points (°C) |
Yields (%) |
Theoretical % (practical %( C H N S Sn |
||||
|
Cephalexin(L) |
Pale Yellow |
198 - 200 |
----- |
55.32 (55.63) |
4.93 (5.54) |
12.10 (11.98) |
9.23 (9.58) |
------- |
|
Ph2SnL2 |
Orange |
194 - 196 |
97 |
54.73 (53.95) |
4.38 (4.90) |
8.70 (7.91) |
6.64 (6.20) |
12.29 (12.95) |
|
Bu2SnL2 |
Red |
125 - 127 |
78 |
51.90 (51.35) |
5.44 (4.95) |
9.08 (9.60) |
6.93 (7.03) |
12.82 (11.95) |
|
Me2SnL2 |
Orange |
158 - 160 |
98 |
48.53 (47.78 ) |
4.55 (5.20) |
9.99 (10.13) |
7.62 (7.15) |
14.11 (13.97) |
Fourier transform infrared Spectroscopy
The ligand's FTIR spectrum (Cephalexin) displays a shift in the main frequency of the hydroxyl group of the ligand's carboxylic acid, which is characterized by a wide absorption at (3400-2400) cm-1. The change also involves the frequency C-O and C=O with wave numbers of 1265 cm-1 and 1685 cm-1 respectively, that are caused by deprotonation of the ligand's carboxylic group as a result of complex formation between the ligand and tin ion [20]. Infrared spectroscopy studies of organotin complexes also showed new absorption bands. These bands are associated with the 449-459 cm-1 and 505-513 cm-1 for (Sn-O) and (Sn-C) resonance regions respectively (Figure 1). The existence of these bands indicates that the Sn (IV) - ligand coordination is stable. The carboxyl, aryl, or alkyl groups connected to the tin center coordinates it [21,22]. The IR data for some of the complexes' unique groups are shown in Table 2.
Table 2: FTIR spectroscopy data of prepared diorganotin-cephalexin complexes (1-3).
|
Complexes |
C=O |
C-O |
Sn-O |
Sn-C |
|
Ph2SnL2 |
1624 |
1188 |
449 |
505 |
|
Bu2SnL2 |
1651 |
1235 |
450 |
511 |
|
Me2SnL2 |
1653 |
1240 |
459 |
513 |

Figure 1: FTIR spectrum of cephalexin and its complexes
Nuclear magnetic resonance Spectroscopy
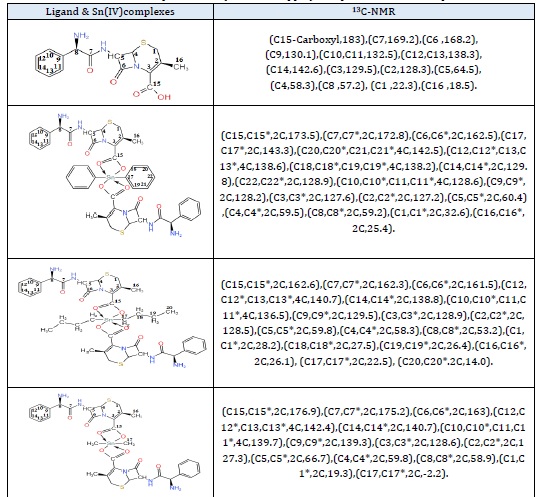
Organotin (IV) moiety, there is a reduced up-field shift for complexes [23]. The increase in the coordination number of the tin atom leads to an increase in chemical shift [24]. N-H proton of ligand appeared as a singlet in all complexes, which proposes the N atom does not coordinate to the tin center. The ligand cephalexin and complexes are shown in table 3 and (Figures S1 and S2) (see supplementary data). The 13C-NMR data of the ligand and their complexes agreed with the 1H-NMR and FT-IR data for compound formation. Because of the decrease in electron density at carbon atoms when oxygen is bound to an electropositive tin atom, the complexes' C15-carboxyl were displaced downfield compared to the position in the ligand. This observation adds to the indication that complexation happened via the carboxylic group's oxygen atoms. As displayed in table 4 and (Figures S3 and S4) (see supplementary data). In 119Sn-NMR of Ph2SnL2, Bu2SnL2 and Me2SnL2 complexes resonating at, - 276.42, -258.75 and -209.97 ppm respectively, It is within the complexes' range of hexa-coordinated organotin (IV) [25,26]. Figure S5 (see supplementary data)depicts the complex Me2SnL2.
Table 3: The 1H-NMR spectra (DMSO-d6; ppm) of cephalexin and its complexes.
|
Ligand and Sn(ΙV)complexes |
1H-NMR |
|
L-Cephalexin |
δ12.91(s,1H,COOH), 8.90 (s,1H,NH), 8.62(d, J=9.6 Hz.2H,Ar), 8.47-7.30 (m,2H,Ar), 7.25(t, J=8.5 Hz,1H,Ar), 5.41 (d, J=10.3 Hz,1H,N-CH), 5.25(d, J=8.9 Hz ,1H,CO-CH), 5.02 (s,1H,PhCH-), 4.56-3.24(br,2H,NH2), 3.21(d, J=13.9 Hz,1H,S-CH), 3.16(d, J=13.9 Hz ,1H,S-CH), 2.09 (s,3H,Me). |
|
Ph2SnL2 |
δ 9.25(s,2H,2NH), 8.63 (d, J=9.5 Hz,4H,Ar), 8.54-7.10 (m,12H,Ar), 5. 88(t, J=5.5 Hz,4H, Ar), 5.59 (d, J=5.0 Hz,2H,2N-CH), 5.34 (d, J=7.5 Hz,2H,2CO-CH),4.97 (s,2H,2PhCH-), 4.45-3.26(br,4H,2NH2), 3.12(d, J=9.5 Hz,4H, 2S-CH2), 2.50(s,3H,2Me). |
|
Bu2SnL2 |
δ 9.15(s,2H,2NH), 8.68(d, J=22.8 Hz,4H,Ar), 8.59-6.92(m,4H,Ar), 6.54(t, J=11 Hz,2H,Ar),5.94(d, J=10 Hz, 2H,2N-CH), 4.96 (d, J=23.5 Hz,2H,2CO-CH) 4.45(s,2H,2PhCH-), 4.18-3.23(br,4H,2NH2) 3.20(d, J=9 Hz,4H,2S-CH2), 2.53 (s,6H,2Me),1.65(qut, J=7.5 Hz, 4H,2CH2),1.45(sex, J=7.5 Hz,4H,2CH2),1.20(t, J=7.5 Hz,6H,2Me), 0.95 (t, J=7.3 Hz,4H,2CH2). |
|
Me2SnL2 |
δ 8.63(s,2H,2NH), 8.06 (d, J=14.5 Hz,4H,Ar),7.98-6.84 (m,4H,Ar), 6.59(t, J=6.5 Hz ,2H, Ar),6.05 (d, J=11 Hz, 2H,2N-CH), 5.49 (d, J=8.3 Hz,2H,2CO-CH), 5.35(s,2H,2PhCH-), 5.20-3.50 (br,4H,2NH2), 3.45(d, J=6.5 Hz,4H,2SCH2), 2.52(s,6H,2Me), 0.72(s,6H,2Me). |
Table 4: 13C-NMR spectral data (DMSO-d6, δ, ppm) of Cephalexin and its complexes.
Antioxidant activity
The antioxidant activity of the ligand cephalexin and its generated complexes was assessed by using the DPPH and CUPRAC procedures. In a microplate reader, an aliquot of the complexes and ligands and a blank without a sample were used. Following the addition of all components, a 0.01 mM DPPH solution was added and absorbance at time zero at a UV wavelength of 490 nm was immediately measured. For 15 minutes, measurements were taken every 5 minutes. Tannic acid was employed as the reference molecule at a concentration of 20 g/mL. The antioxidant activity of complexes and ligands was measured as a decline in DPPH methanolic solution absorbance induced by the impact of each complex or ligand's activity to donate hydrogen, allowing the reduced form of the DPPH radical (pale yellow solution) to emerge [27-35]. Metallic compounds have the antioxidant activity in studies due to the metal moiety, which improves their activity as a result, organotin compounds are predicted to have antioxidant activity due to the presence of the metal moiety [36,37]. Organotin complexes outperform ligands in terms of antioxidant activity against the stable free radical. This is mainly due to the overall biological effect of organotin chemicals. In spite of their inherent toxicity, the organotin (IV) complexes have antioxidant properties [38-41]. Complex 3 (Me2SnL2) exhibits a larger proportion of scavenging behavior in both DPPH and CUPRAC methods, as presented in tables 5, 6 and (Figures 2, 3). This is related to the stability of the symmetric compound. Furthermore, methyl complex has more content than others, resulting in greater antioxidant activity [42]. Therefore, antioxidant activity of di-organotin (IV) complexes took the following order: Me2SnL2 > Bu2SnL2 > Ph2SnL2.
Table 5: The result of antioxidant activity of ligand and its complexes by DPPH method.
|
Control Absorbance=0.378 λ=490 nm |
||||||
|
Compounds |
After 5 min |
After 10 min |
After 15 min |
|||
|
|
Sample Abs. |
% Inhibition |
Sample Abs. |
% Inhibition |
Sample Abs. |
%Inhibition |
|
Cephalexin |
0.236 |
37.566 |
0.233 |
38.360 |
0.232 |
38.624 |
|
Ph2SnL2 |
0.201 |
46.825 |
0.19 |
49.735 |
0.148 |
60.847 |
|
Bu2SnL2 |
0.185 |
51.058 |
0.159 |
57.937 |
0.142 |
62.434 |
|
Me2SnL2 |
0.183 |
51.587 |
0.153 |
59.524 |
0.132 |
65.079 |

Figure 2: DPPH method of cephalexin and its complexes at T=5, 10 and 15 min.
Table 6: The antioxidant activity of cephalexin and its complexes by CUPRAC method.
|
Control Absorbance=0.264 λ=490 nm |
||
|
Compounds |
Sample Abs. |
% Inhibition |
|
Cephalexin |
0.224 |
15.151 |
|
Ph2SnL2 |
0.192 |
27.273 |
|
Bu2SnL2 |
0.164 |
37.879 |
|
Me2SnL2 |
0.152 |
42.424 |

Figure 3: CUPRAC method of cephalexin and its complexes activity.
Conclusion
Condensation reaction with cephalexin yielded suitable di-organotin (IV) complexes. To identify the produced compounds, several techniques (IR, 1H, 13C and 119Sn NMR) and exact element analysis were used. Furthermore, during the DPPH and CUPRAC procedures, antioxidants were used. All synthesized complexes have a higher antioxidant activity than ligand-derived complexes. According to the results, the compound Me2SnL2 performed the best in both procedures.
Acknowledgment
The authors thanks university of Babylon for support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all aspects of this work.
Conflict of Interest
The author declared that they have no conflict of interest.
ORCID:
Angham G. Hadi
https://orcid.org/0000-0002-5396-7560
Supplementary File
Additional supporting information related to this article can be found, in the online version, at file:///C:/Users/Dr.zynab/Downloads/Supplementary%20File%20(1).pdf
HOW TO CITE THIS ARTICLE
Rafid Ryyis Arraq, Angham G. Hadi. Synthesis, Identification, and Anti-oxidant Activity of Di-Organotin (IV)-Cephalexin Complexes. J. Med. Chem. Sci., 2023, 6(2) 392-401


