Document Type : Original Article (Special Issue)
Authors
1 College of Science, University of Thi-Qar, Iraq
2 College of Dentistry, University of Thi-Qar, Thi-Qar, Iraq
Abstract
The PVA@WNS composite has been successfully prepared by attaching the polyvinyl alcohol and Walnut shell powder with (1:1) ratio from plant and has been analyzed by using FT-IR, SEM, and AFM, and then it was used as an adsorbent of methylene blue dye from synthetic wastewater. The adsorption of MB dye on the PVA@WNS composite was studied as a function of contact time and concentration. Isotherm adsorption was also described by the Langmuir and Freundlich models. It was found that Langmuir isotherm (R2= 0.933) is better than the Freundlich model (R2= 0.925) which implies that the MB adsorption on PVA@WNS composite was monolayer. Thus, qmax calculated from the Langmuir model was found to be 5.938 mg.g−1 and was consistent with the experimental data. The synthesized PVA@WNS composite showed an excellent activity towards the removal of methylene blue from synthetic wastewater.
Graphical Abstract
Keywords
Main Subjects
Introduction
Dyes are synthetic pollutants found in aquatic sources where could be harmful to human life. Some health issues are related to dyes effect such as urinary bladder cancer, toxicity to cells like kidney, liver, and irritated skin [1]. So, the removal of dyes has become an essential target in obtaining clean water systems. There are three main types of dyes such as cations, anions, and natural dyes [2,3]. Methylene blue is fully recognized dye that has been used in wide number of industries, so it was selected as a model to measure removal possibility from specific water sources like wastewater. It is a cationic dye (known also as thiazine) and has been extensively used to color papers, cotton, wool, and many other industries. When inhaled, MB may cause serious health issues, for example, vomiting, nausea, and breathing difficulties. It is worth mentioning that methylene blue has only recently causes severe central nervous system toxicity [4]. In addition, central nervous system in the brain can be influenced by MB over dosage. The reasons mentioned above have made it crucial to remove MB from the environment [5]. A number of techniques have been employed to remove dyes such as ion exchange, photocatalytic, membrane separation, chemical oxidation, and adsorption methods. Adsorption among them were found to be simple, low cost, and efficient for water treatment [5,6]. It has been extensively applied due to cause no harm to the nature [7,8]. Adsorption shows multiple benefits according to the use of natural and synthetic materials as potential adsorbents to treat different water sources [9,10]. The disadvantages of some adsorbents can be summarized as expensive, having low capacity, and not showing high or same effectiveness towards pollutants [11, 12]. Therefore, adsorption method has been considered as an excellent method to overcome previously mentioned drawbacks. It is becoming quite common and widely used by scientists around the world to remove different pollutants from water media [13-15]. Among all conventional and advanced materials which have excellent adsorptive properties such as inorganic and organic products usually used as adsorbents, natural waste materials are quite promising and attractive for this purpose [16-19]. They paly crucial roles as alternatives to synthetic adsorbents, natural waste adsorbents reveal capability to be prepared in large amounts, affordability, and safe usage. For example, cellulose fibers made from newspapers-waste [20], waste coffee can be grounded to obtain fine powder [21], wastes from algerian olive oil cake [22], cotton waste from flowers [23], and peeled Egyptian luffa [24,25] are useful too. WS is an example of promising adsorbent which were produced in different countries. It is hard and brown from the outside (shell), and also good for human health and great source for different nutrients and vitamins [26]. In current study, polyvinyl alcohol (PVA) is used as a modifier to walnut shell powder (PVA @WNS composite) to study removal efficiency of MB dye.
Materials and Methods
The PVA @WNS composite were recorded by instrumentation in the Table 1.
Table 1: Instrumentation used in this study
|
Device information |
Instrumentation |
|
Shimadzu, Japan |
The Fourier transform infrared spectra (FT-IR) |
|
TESCAN MIRA3 FRENCH |
Scanning Electron Microscopy (SEM) |
|
(NaioAFMNanosurf Switzerland) |
Atomic Force Microscopy (AFM) |
Walnut plant and eggs (adsorbents) were purchased from Local Market. Methylene blue MB (C16H18ClN3S) (adsorbate) was obtained from Sigma Aldrich.
Preparation of Walnut Shell Micro-Powders
First, original walnut plant was cracked separately into small parts and washed several times with water tap, and then it was washed with distilled water to get rid of impurities and dusty particles. Next, the cracked walnut shell was heated up at 105 °C to be perfectly dried. The plant was then grinded at home by using a home grinder. The grinded powders were sieved individually using a 75 µm sieve (hole’s diameter). Powders are ready now to be used for dyes adsorption experiments.
Preparation of PVA@WNS composite
PVA@WNS composite was prepared with mixing 0.1 g of WNS and 0.1 g of PVA by using ultrasonic vibration for 90 min at 50 °C. The product poured into a glass mold and kept for 24 hours at room temperature. Then, dehydrate in oven at 70 °C for 3 hours [27,28] (Scheme 1).

Scheme 1: Preparation of PVA@WNS composite and using for removal methylene blue dye
Batch experimental studies
The adsorption capacity of methylene blue dye in aqueous solutions was performed by a batch absorption studies. The amount of MB dye adsorbed on the adsorbent was determined from difference initial and equilibrium concentrations by UV–Vis at 660 nm. Batch adsorption experiments were conducted to investigate the effect adsorption time of (30-40 hours), initial MB concentration of (5–40 mg/L) and weight of composites (0.05–0.25 g). The qe (mgg-1) and the %R of blue dye on PVA@WNS composite were expressed with the following equations [29]:
Where,
![]()
![]()
C0(mg/L): initial concentrations of MB dye in the solution,
Ce (mg/L): equilibrium concentrations of MB dye in the solution,
V (L): the volume of the solution,
W (g): the mass of adsorbent used.
To investigate the adsorption isotherm, two models of Freundlich and Langmuir isotherms equations were applied (see Table 2).
Table 2: The adsorption isotherm models
|
Isotherms |
Equations |
References |
|
Freundlich isotherm |
[41-43] |
|
|
Langmuir isotherm |
[41-43] |
Results and Discussion
FT-IR analysis
Figure 1 displays the FT-IR spectrum of PVA@WNS composite. The 3425–3465 cm−1 peaks are attributed to –OH stretching vibration of hydroxyl functions PVA and/or physisorbed water on the WNS surface [30,31]. The peak at 2929 cm−1 is assigned to C-H vibration presented in -CH2-and -CH3. The presence of the bands at about 1739 and 1646 cm-1 can be attributed to the C = C stretching vibration of aromatic carboxyl groups in the walnut shell [28]. Finally, the broad band between 1115 and 1042 cm-1 ascribed to C–O peaks of (R–OH) groups in alcohol of walnut shell and PVA [32].

Figure 1: FTIR spectra of PVA@WNS composite
FESEM analysis
The SEM images of PVA@WNS composite (Figure 2) indicates that the PVA@WNS surface was soft and compacted and the separation of two components phases (PVA and WNS) was not found, this showed a clear compatibility and uniform morphology between components in the composite. The SEM micrographs of PVA@WNS were taken at 1 𝛍m (low resolution) and 200 nm (high resolution), as depicted in (Figure 2(a,b)).

Figure 2: FESEM images of PVA@WNS composite
AFM analysis
AFM technique was used to determine the surface topography, roughness, and grain size of the prepared composite. Figures (3) and (4) represent the captured AFM images and the grains diameters histogram for PVA@WNS composite. The AFM results show that the composite grains mean diameter is 26.47 nm which supply suitable surface area for adsorption processes. In addition, the PVA@WNS composite roughness parameters, Sa (mean roughness) and Sq (root mean square roughness) were 8.5 nm and 13.5 nm, respectively.

Figure 3: AFM images of PVA@WNS composite: 3 dimensions (left), 2 dimensions (right)

Figure 4: Grains’ diameters histogram of PVA@WNS composite
Dye removal studies
Effect of contact time
Figure 5 indicates the role the shaking time on adsorption capacity of MB dye on surface PVA@WNS composite. The result showed that the adsorption process is faster in the starting point, but subsequently it become slow due to the sorption sites occupied with adsorbate [33,34].

Figure 5: Effect of contact time on removal methylene blue by PVA@WNS composite
Effect of concentration
Figures 6 and 7 illustrate the effect of initial MB concentration on its adsorption capacity and the percentage of dye removal by using different concentrations of dye solution (5 mgL-1, 10 mgL-1,
20 mgL-1, 30 mgL-1, and 40 mgL-1). It was found that adsorption capacity of MB dye increased with maximizing the initial concentration of dye, while %R of dye decreases with increasing the C0 of dye. [33,34].

Figure 6: Effect of initial concentration of methylene blue in the solution on capacity sorption of methylene blue onto PVA@WNS composite

Figure 7: Effect of initial concentration of methylene blue dye in the solution on efficiency adsorption of methylene blue onto PVA@WNS composite
Adsorption isotherm
The adsorption studies were conducted by varying the initial MB dye concentrations (5, 10, 20, 30, and 40) ppm with a constant weight of adsorbent (0.25 g) and optimum shaking time for capacity adsorption of MB dye in this study. The Langmuir and Freundlich isotherms were shown graphically in Figures 8 and 9 and the corresponding parameters were listed in Table 3. Depending to the coefficients of correlation acquired from linear regression, it was found that in all cases the Langmuir model were more favorable data than that of Freundlich model because the (R2) values are higher for Langmuir isotherm than that for the Freundlich isotherm [33-35].
Table 3: Parameters of Langmuir isotherm and Freundlich isotherm models
|
T(K) |
Langmuir Model |
Freundlich Model |
||||
|
Qmax(mg.g-1) |
KL |
R2 |
N |
Kf |
R2 |
|
|
298 |
5.938 |
0.067 |
0.933 |
1.204 |
0.638 |
0.925 |
Table 4: Comparison of maximum adsorption capacity of PVA@WNS composite some other adsorbents
|
Adsorbent |
Qmax (mg.g-1 ) |
Ref. |
|
Coir pith as adsorbent |
5.8 |
36 |
|
Apricot stones as adsorbent |
4.133 |
37 |
|
Silk cotton hull as adsorbent |
2.40 |
38 |
|
Sago waste as adsorbent |
4.51 |
38 |
|
Banana pith as adsorbent |
4.67 |
38 |
|
Maize cob as adsorbent |
5.00 |
38 |
|
PANI base/silica composite as adsorbent |
4.75 |
39 |
|
PVA@WNS composite as adsorbent |
5.938 |
In this study |
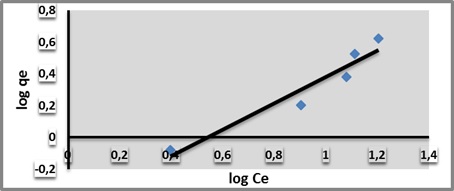
Figure 8: Freundlich adsorption isotherm of methylene blue dye onto PVA@WNS composite

Figure 9: Langmuir adsorption isotherm of methylene blue dye onto PVA@WNS composite
Conclusion
The data of this paper indicates that The PVA@WNS composite can be successfully utilized for removal of methylene blue dye from an aqueous solution. The methylene blue dye adsorption was tested at different conditions such as contact time and concentration. Isotherm adsorption was also described by the Langmuir and Freundlich models. The sorption of the methylene blue dye by the PVA@WNS composite followed a monolayer sorption model Langmuir isotherm rather than multilayer model for the Freundlich model. Thus, the maximum adsorption capacities calculated from the Langmuir model was found to be 5.938 mg.g−1 and was consistent with the experimental data.
Acknowledgment
I would like to extend my deep appreciation and sincere thanks to Dr. Samah Hussein Kadhim. The same goes to technical staff at the department of chemistry for providing the necessary technical assistance and support in the experimental.
Funding
This research did not receive any specific grant from fundig agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
There are no conflicts of interest in this study.
ORCID:
Samia Mezhr Merdas
https://www.orcid.org/0000-0002-1077-7556
HOW TO CITE THIS ARTICLE
Samia Mezhr Merdas, Wed Al-Graiti, Aliaa Shakir Abd Al-Ameer, Using PVA@WNS Composite as Adsorbent for Methylene Blue Dye from Aqueous Solutions. J. Med. Chem. Sci., 2022, 5(7) 1289-1298
https://doi.org/10.26655/JMCHEMSCI.2022.7.18
URL: http://www.jmchemsci.com/article_154742.html


