Document Type : Original Article
Authors
Baghdad University, College of Science, Department of Chemistry, Baghdad, Iraq
Abstract
New π-conjugated system compounds were synthesized by pyromellitic diimide core coupled with side chain containing 1,2,4-triazole rings. The new compounds were characterized by some physical properties, FT-IR and 1H-NMR and the compounds showed a high melting point with some above 300 °C depending on high intramolecular attractive and the Vander Waals attraction between various substituent groups. The optical properties of the prepared compounds were investigated by UV–vis measurement and optical energy gap were estimated by about 2.85-4 e.v. Electro physical properties showed the compounds to behave as p-type semiconductor with acceptable mobility.
Graphical Abstract
Keywords
Main Subjects
Introduction
Pyromellitic diimide (PMDI), as considered as the smallest of aromatic diimides, has received significantly less interest than the analogue naphthalene and perylene diimides in spite of present favorable attitude in electronic settings [1-3]. To meet the requirements for the forward implementation in the intense environment, the mechanical properties of polyimides and diimides need to be highly afflicted [4] while maintaining the good thermal properties and chemical resistance, which is also a duty and importance of research at all times [5-7].
1,2,4- triazole five-membered heterocyclic is one of the most common heterocycles. Triazoles have been shown to have confirmed eligible characteristics, like reduction conditions, high acid-base hydrolysis stability and metabolic degradation-resistance [8,9]. They include electrical, mechanical, magnetic, optical and corrosion properties far better than their individual components, because of their electro-active and conductive nature [10-13].
Materials and Methods
Chemicals and instruments
- Infrared spectra were recorded using Fourier Transform infrared SHIMADZU (8300) (FTIR) infrared spectrometer, Japan, KBr disc in the (4000-600) cm-1. Spectral range was performed by Baghdad university /college of science/ chemistry department
- 1H-NMR and 13C-NMR spectra were recorded in Iran using recorded on EGELENT, Ultra shield 500MHz using tetramethyl silane as internal standard and (DMSO-d6 or dis. Water) as a solvent.
- Uv-vis scan was scanned by UV spectrophotometer SHIMADZU (UV-1800)
- All chemicals were used sullied from BDH, Alpha and Merk.
Synthesis of N,N'- bis (2-amino acetyl chloride) pyromellitic diimide (2,14,15)
N,N'-bis amino pyromellitic diimide (5 mmol, 1.23 g) was added to chloro acetyl chloride (1-2 mL) and refluxed on steam bath for 2-3 h; after cooling, yellow precipitate was formed, dried and recrystallized by benzene (Scheme 1).
Synthesis of N,N'-bis (2-amino acid hydrazide) pyromellitic diimide (3,16)
A mixture of the N,N'-bis (2-amino acetyl chloride) pyromellitic diimide (2) and hydrazine hydrate (0.015 mol, 0.75 mL) in ethanol (50 mL) was refluxed for 10 h. The reaction mixture was allowed to cool and the separated product was filtered and dried. Crystallization of the crude product was conducted with benzene (Scheme 1).
Synthesis of shiff base: N, N'-bis[2-amino acet hydrazine benzylidene]-pyromellitic diimide (4-10)
2 drops of glacial acetic acid (0.1 mL) was added to different derivatives of aldehydes (2 mmol) and refluxed for 15-20 min, then N,N'-bis (2-amino aceto hydrazide) pyromellitic diimide compound (3) (0.4 g, 1 mmol) was added and refluxed for 3-6 h, controlled by TLC; the product was dried and washed with ether, recrystallized from di ethyl ether and petroleum ether (Scheme 1).
Synthesis of N,N'- bis 1,2,4-triazole pyromellitic diimide (11-17)
Benzonitrile (0.2 mL, 2 mmol) was added to Shiff basses compounds (11-19) dissolved in (butanol or ethanol) in presence of K2CO3 and refluxed for 24 h. The product was dried and recrystallized from petroleum ether (Scheme 1).
Results and discussion
In this study, new 1,2,4- triazole heterocyclic compounds based on pyromellitic diimide core were prepared in 4 steps from N,N'- bis amino pyromellitic diimide, initially reacting with chloro acetyl chloride and getting heated on a steam bath then reacting with hydrazine hydrate 99%. Then, at step 3 it reacted with different aromatic aldehyde by Schiff base reaction. Step four included synthesizing a factional group 1,2,4- triazole by reacting with benzo nitrile in presence of potassium carbonate.
The physical properties of prepared compounds are listed in Table 1.

Scheme 1: The chemical steps for the synthesis of compounds (1-17)

The FT-IR spectrum (Table 2) for compounds (11-17) showed a stretching new band in range of 1446-1521 cm-1 due to N-N triazole and N-H amide appearance at the range of 3159-3286 cm-1. A hard-stretching band at rang 1618-1677 cm-1 refers to carbonyl imide and weak band at range 1699-1766 cm-1 (17) due to carbonyl amide. Other specific bands are listed in Table 2.
Table 2: characteristic IR absorbtion data compounds (11-17)
|
Compound NO. |
n (NH) |
n(C-H) aromatic |
n(C-H) aliphatic |
n(C=C) aromatic |
n(C=O) Imide amide |
n (N-N)triazole ring |
n(C-N) |
|
11 |
3240 |
3068 |
2937,2806 |
1595,1552 |
1710 1629 |
1446 |
1350 |
|
12 |
3276 |
3026 |
2875,2870 |
1558,1544 |
1699 1618 |
1508 |
1373 |
|
13 |
3251 |
3050 |
2925,2902 |
1487,1402 |
1743 1652 |
1562 |
1369 |
|
14 |
3286 |
3033 |
2910,2840 |
1550,1461 |
1714 1677 |
1517 |
1379 |
|
15 |
3191 |
3041 |
2979 |
1425-1575 |
1728 1658 |
1521 |
1363 |
|
16 |
3159 |
3060 |
2977,2927 |
1577,1508,1492 |
1776 1652 |
1512 |
1336 |
|
17 |
3201 |
3043 |
2891,2937 |
1512,1573 |
1740 1658 |
1450 |
1326 |
Compounds (15) and (17) were characterized by 1H-NMR. Compound (15) in the 1H-NMR spectrum of (Table 3) showed signals at δ 6.26 ppm for (s, 4H, OH), at δ 8.27 ppm for (s, 2H, NH) and δ (7.14-10.15) ppm for (m, 18H, Ar-H) where compound (17) showed signals at δ2.55 ppm for (s, 6H, CH3), at δ (6.26-10.73) ppm for (m, 20H, Ar-H), at δ 8.77 ppm for (s, 2H, NH).

Application
Optical properties
The UV scan for compounds showed λmax for compound 15 at 434,411, respectively, and λmax at 327,252 nm, respectively for compound 16.

Figure 1: UV-VIS for compound 15

Figure 2: UV-VIS for compound 17
The estimated direct energy gaps [18] are listed in Table 4.
The electro physical characteristics were calculated by Hall Effect [19] for compound 15 showed p-type behavior and other electro physical characteristics are listed in Table 5.
Table 4: estimated direct energy gaps (18)
|
Comp. |
λmax |
Eg e.v |
|
15 |
434 |
2.85 |
|
|
411 |
3.017 |
|
17 |
327 |
3.79 |
|
|
252 |
4.92 |
|
parameter |
Value |
|
|
Conductivity |
3×10-7 |
1/Ω cm |
|
Mobility |
0.39 |
Cm2/Vs |
|
Resistivity |
3.28×106 |
Ω Cm |

Figure 3: Hall effect parameter
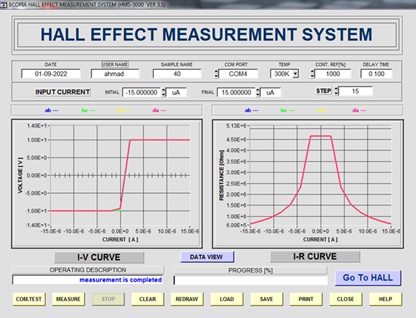
Figure 4: I-V & I-R curves for compound (15)
Conclusion
New 1,2,4- triazole heterocyclic compounds based on pyromellitic diimide core were synthesized. This compounds were characterized by FT-IR and 1H-NMR. The optical properties of the prepared compounds were investigated by UV–vis measurement and optical energy gap were estimated by about 2.85-4 e.v. The electric physical experiment of the compounds can be used in solar cell as p-type semiconductor.
Funding
This research did not receive any specific grant from fundig agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
There are no conflicts of interest in this study.
ORCID:
Ahmed Khudhair Kadhim
https://www.orcid.org/0000-0002-9387-4051
HOW TO CITE THIS ARTICLE
Ahmed Khudhair Kadhim, Muna Ismael Khalaf. A Core-Extended Pyromellitic Diimide as a P-Channel Semiconductor. J. Med. Chem. Sci., 2023, 6(1) 62-70


