Document Type : Original Article
Authors
Department of Pharmaceutical Chemistry, College of Pharmacy, University of Mosul, Ninavah-41002, Iraq
Abstract
There is an unequivocal relationship between oxidative stress and diabetes development, progression, or both. In recent years, some novel bioactive compounds derived from plants have shown antidiabetic activity with more efficacy than the oral hypoglycaemic medications used in clinical therapy, implying a bright future for diabetes treatment. In this work, the powdered quince dried seeds were subjected to extract with ethanol, ethyl acetate, chloroform, n-hexane, and diethyl ether using sonication-catalyzed extraction. Then, a set of tests were used to conduct preliminary phytochemical screening. In vitro, hydroxyl radical- and DPPH radical-snaring assays were used to assess the antioxidant activity of the acquired extracts. The inhibition assays for α-glucosidase and α-amylase were used to determine the antidiabetic efficacy of the extracts. Compared to ethanol extract, ethyl acetate, chloroform, n-hexane, and ether extracts had weaker free radical-snaring, α-amylase, and α-glucosidase inhibitory activities. The results of this study revealed that the crude extracts of quince seeds are good sources of key phytochemical components with significant antioxidant and antidiabetic properties and can be considered a promising source of lead compounds for the management of diabetes mellitus.
Graphical Abstract
Keywords
Main Subjects
Introduction
Diabetes is one of the most pressing medical issues confronting society in the 21st century. It was estimated that diabetes affected 425 million individuals globally in 2017, with the number expected to rise to 629 million by the middle of the century due to its epidemic prevalence [1,2]. Diabetes mellitus (DM) is an endocrine and metabolic ailment defined by its chronic nature, hyperglycaemia, and diverse vascular consequences. In addition, the underlying pathophysiology is characterized by insulin insufficiency, insulin insensitivity, or both. Many studies have demonstrated that oxidative stress has a fundamental role in the underlying molecular mechanisms related to the evolution and progression of DM and its complications, much as it does in other pathologies like cancer and neurodegenerative illnesses [3–5].
In addition to mitochondrial dysfunction and impairment of the conventional insulin signalling pathways, the oxidative stress can induce the DM development via three more major molecular mechanisms, including induction of inflammatory responses, β-cell dysfunction, and finally, through glucose transporter-4 (GLUT-4) down-regulation, localization, or both [6].
The protracted use of oral hypoglycaemic medications and/or insulin in diabetic patients causes a variety of health risks, including oedema, gastrointestinal issues, hypoglycaemia, as well as hepatic-renal disorders. Natural products can provide a richness of glycaemic-controlling chemicals or scaffolds with hypoglycaemic impaction. Thus, plant-derived products with an antidiabetic potential can be exploited as a substitute for co-existing diabetes medications with minimal harmful impact and low medical charges [7,8]. Furthermore, the antioxidant properties of most of these plant-derived products can be used to combat the substantial growth in oxidation load and dramatic drop in redox balance which emerges with DM [9,10].
Quince (Cydonia oblonga Mill., C. oblonga, Figure 1) is a member of the Cydonia genus, which would be a part of the Rosaceae family, subfamily of Pomoideaeis. C. oblonga is well-known for its nutritive and ornamental properties. The fixed focal seeds are designed in two vertical rows in the bright yellowish quince fruit [11,12].

Figure 1: The physical appearance of the fruit named Cydonia oblonga
Various bioactives of C. oblonga fruit have been extensively studied, and mostly to its antioxidant, antibacterial, and anti-ulcerative properties, this fruit phenotype is now extensively regarded as a valuable, low-cost, and easily accessible nutritional source of health-promoting chemicals. Seeds, on the other hand, have received far fewer studies [13,14].oblonga’s seeds have traditionally been used to treat dysentery, intestinal colic, diarrhoea, sore throats, coughs, and bronchitis [15]. Recently, Carvalho et al. discovered that C. oblonga seeds are a good source of the plant-derived products with a substantial antiproliferative activity on renal cancer cells [16]. In addition, the anti-inflammatory efficacy of seeds’ crude extract has been researched and found to be markedly effective compared with aspirin. Furthermore, studies revealed that the seeds of C. oblonga extract has a promising antimicrobial activity against Enterobacter aerogenes, Enterococcus faecalis, S. aureus, P. aeruginosa, and Candida albicans [17, 18].
As the antidiabetic properties of C. oblonga seeds’ extracts have only rarely been examined, this study aimed to estimate the in-vitro free radical-snaring and antidiabetic potentials of five different sonication-acquired extracts of C. oblonga seeds. The extraction was conducted using sonication-catalyzed methodology, and the resulting extracts were subjected to a variety of phytochemical assays in order to evaluate the presence of certain plant-derived primary and secondary metabolic products.
Materials and Methods
The employed solvents and chemicals in this study were provided from Sigma-Aldrich and Tokyo Chemical Industry. The dried seeds of C. oblonga were purchased from a public market in Mosul, and then they were taxonomically distinguished by consultants from the College of Agriculture and Forestry/University of Mosul. A water bath supplied with a sonication source (40 kHz-Power sonic 410, 350 W, Southern Korea) was employed for catalyzing the extraction process.
Processing of C. oblonga Seeds’ Extract
The seeds of C. oblonga were crushed in a coffee grinder and sieved into a fine powder. For the extraction of the seeds’ powder, five solvents of different polarities were used individually. Twenty-five grams of the seeds’ powder were mixed with a quarter liter of each solvent. The extraction was carried out using the sonication-catalyzed extraction (SCE) methodology, in which the resultant blends were irradiated for 30 minutes at 30 °C in a water bath supplied with a sonication source (40 kHz). The sonicated mixture was filtered through Number 1 Whatman filter paper, and the residual solvents were concentrated to dryness under the reduced pressure [19].
Certification of the Plant-Derived Primary and Secondary Metabolic Products
Twenty milliliters of each extractant were combined with two grams of the C. oblonga seeds’ powder. After its sonication, the blend was filtered and phytochemically inspected for the presence of various plant-derived primary and secondary metabolic products. Those included betacyanins, anthocyanins, emodin, terpenoids, flavonoids, carbohydrates, coumarins, tannins, alkaloids, phenols, steroids, proteins, glycosides, saponins, and amino acids using Harborne’s widely acknowledged procedures [19–21].
Free Radical-Snaring Assays
The ability of the sonication-acquired extracts of C. oblonga seeds to snare the reactive hydroxyl groups and DPPH (1,1-diphenyl-2-picryl-hydrazyl) free radicals, in addition to the electron donation in a redox reaction, was collectively investigated using L-ascorbic acid (Vit. C) as a standard.
From the originally extracted mixture (1000 mg/mL), solutions of the following eight concentrations were prepared using the identical solvents applied in the extraction: 1000, 750, 500, 250, 125, 100, 50, and 25 μg/mL. Various diluted concentrations of Vit. C have been used, including 200, 100, 50, 25, 12.5, and 6.25 μg/mL using ethanol as a diluent. For each admixture phenotype, the snaring percentage (S%) values for the involved concentrations were estimated using the following mathematical equation:
The symbols Abssample and Abscontrol, respectively, denote the absorptions of the tested samples and the control substance at a specific visible wavelength.
The snaring capability of the sonication-acquired extracts of C. oblonga’s seeds, denoted by SC50, is defined as the concentration of the extracts sufficient to reduce half of the oxidized iron particles or snare half of the free radicals. This metric depicts the link between the values of S% and the corresponding concentrations expressed in the logarithmic scale employing non-linear regression. [22].
DPPH Snaring Assay
The sonication-acquired extract of C. oblonga’s seeds (1.5 mL) was mixed with 0.5 mL of the 0.1 mM ethanolic DPPH solution at a particular concentration. Aluminum flakes were employed to shelter the combined solution from daylight. Then, the wrapped system was preserved for half an hour at 25 °C, whereas the control solution contained 0.5 mL of ethanolic DPPH solution + 1.5 mL of the extractant. The ability of sonication-acquired extract of C. oblonga’s seeds to modify the DPPH violet hue was measured colorimetrically at 517 nm [23].
Hydroxyl Reactive Moiety-Snaring Assay
Potassium phosphate buffer (0.2 M, pH 7.8, 2.4 mL) was combined with the defined concentration of the sonication-acquired extract of C. oblonga’s seeds (1.5 mL). Ferric chloride, pyridino[3,2-h]quinolone, and hydrogen dioxide were then added in the following volumes: 60 μL (0.001 M), 90 μL (0.001 M), and 150 μL (0.17 M), respectively. After that, the mixture was calorimetrically inspected at 560 nm after being preserved for 5 minutes at 25 °C before. The blank encompasses the above components; while the extracted mixture was substituted with the solvent phenotype utilized in the extraction. [24].
Total Reducing Capacity
Aqueous potassium ferricyanide solution (2mL, 1 %) and 2 mL of 0.2 M PPB (potassium phosphate buffer) adjusted at pH 6.6 were mixed with 1 mL of the defined concentration of the sonication-acquired extract of C. oblonga’s seeds. For 20 minutes, the combination was kept at 50 °C in an electrothermal digitalized water bath. Afterwards, the interaction was halted by applying 2 mL of TCAA (aqueous trichloroacetic acid) aqueous solution (10 %). The resultant was subjected to centrifugation at 2000 rpm for 10 min. A 2 mL supernatant was mixed with 2 mL H2O and 0.4 mL of 0.01% aqueous ferric chloride solution. After a 10-minute incubation period (at 25 °C), the colorimetric assessment of the mixture was accomplished at 700 nm. On the other hand, the blank was prepared with the same ingredients as the testing solution, except that the solvent phenotype used in the extraction replaced the sonication-acquired extract of C. oblonga’s seeds [25].
Evaluation of Antidiabetic Efficacy
In vitro inhibition efficacy of sonication-acquired extracts of C. oblonga’s seeds against two enzyme phenotypes, being well-known crucial in glucose absorption and blood level regulation, porcine α-amylase and yeast α-glucosidase, was investigated. The concentration of the examined mixture which is necessary to inhibit 50% of the enzyme’s activity under test conditions, denoted as IC50 (inhibitory concentration), is a metric used to describe the efficacy of the extracts in these two assays, various concentrations were prepared from the parent extract mixture (1000 mg/mL). The concentrations of 1000, 750, 500, 250, 125, 100, 50, and 25 µg/mL were generated with the solvent employed in the extraction. Each solution (2 mL) was evaporated, and the concentrate was re-dissolved in DMSO to achieve the original pre-evaporation concentration [26].
Assay for Yeast α-Glucosidase Inhibition
A phosphate buffer (PB) solution containing α-glucosidase enzyme (0.1 unit/mL) was combined with a particular concentration of the examined solution in an equal volume of each (20 μL). The prepared extract-enzyme mixture was incubated with a substrate solution (40 μL) at 37 °C for half an hour. It is worth noting that 4-nitrophenyl-α-D-glucopyranoside was dissolved in a PB solution adjusted to pH 6.8 to prepare the substrate solution at a final concentration of 375 μM. The addition of a PB solution containing Na2CO3 (80 μl, 0.2 M) to the tested mixture halted the interaction from progressing. In this assay, acarbose was used as a standard, and the blank was made in the same way as the tested mixture, whereas DMSO was used instead of the extracted mixture. The extracted ability of the solution to inhibit enzyme activity was measured using a colorimetric method at 405 nm, whereas the following mathematical equation was used to calculate the percentage of enzyme inhibition [27]:
α-Glucosidase inhibitory %=Abscontrol – Abssample ÷ Abscontrol×100
Assay for Porcine α-amylase inhibition
In this enzymatic assay, two types of solutions were adopted. The first solution contained 20 µL of alpha-amylase enzyme (2 units/mL) in PB and 20 µL of the investigated sample at a pre-set concentration. The second solution was the substrate mixture which was all set by dissolving the starch in a PB solution (pH 6.8) to acquire 2 mL of 500 µg/mL. Eventually, an equal volume (40 µL) of the two admixes was combined, and then incubated for 10 minutes at 25 °C. An aqueous solution of NaOH (2 mL, 0.4 N) containing anhydrous sodium potassium tartrate (12%) and 3,5-dinitrosalicylic acid (1%) charged of interrupting the ongoing reaction in the incubated admix. Then, the finished mixture was heated for only a quarter of an hour in a boiling water bath, and after that it was cooled down to 25 °C in an ice chest after being adjusted with H2O to 10 mL. The same method by which the incubated solution was prepared was further adopted for the preparation of the blank, except that DMSO was used instead of the extracted mixture, while the standard which was employed in this assay was acarbose. The ability of the extracted solution to inhibit enzyme activity was measured using a colorimetric method at 405 nm, whereas the ensuing mathematical equation was used to calculate the percentage of enzyme inhibition [28]:
α-Amylase inhibitory %=Abscontrol -Abssample ÷Abscontrol ×100
Results and Discussion
Screening the bioactivities and biologically active chemicals of natural products is one of the most common ways to discover the new lead compounds [29]. Many studies have been conducted on the seeds of plants in the Rosaceae family, revealing a diverse spectrum of promising chemical elements and a distinct phenolic profile [19, 20, 30], inspiring this study to scrutinize the anti-hyperglycemic and antioxidant potentials of C. oblonga’s seeds.
Qualitative Phytometabolic Product Screening
Phytoingredients of crude medicinal agents were found to possess a wide range of applications in the management of chronic illnesses. Plants have different phytochemical constituents in terms of both quantity and quality. The chemical composition of plant products can be approximated using a qualitative assessment with the natural crude extract. As a result, preliminary phytochemical analysis aids in perceiving the medicinal value of C. oblonga seeds’ extracts [31–35].
The SCE approach was utilized for extracting C. oblonga’s seeds using five different solvents. Several phyto-metabolites’ existences were investigated via various qualitative phytochemical screening assays on the five crude extracts, as presented in Table 1. The ethanolic extract revealed the superior contents of phytoconstituents compared to the remaining extracts, where the positive results of the tests included carbohydrates, phyto-steroids, phenolic compounds, alkaloids, amino acids, proteins, tannins, and coumarins, which is consistent with the findings of the study conducted by Shaida et al.[18]. The n-hexane extract, on the other hand, was determined to have the least richness in the screened elements.
Evaluation of Antioxidant Activity
In the last few decades, researchers has focused their attention on oxidative stress, as an influential factor in the pathomechanism of diverse diseases including cancer, osteoarthritis, atherosclerosis, diabetes, and cardiovascular disorders [19, 36, 37]. In the case of diabetes, the earlier experimental and clinical studies have suggested that oxidative stress performs a crucial function in the underlying pathogenesis and progression of diabetic problems [38-40]. As a result, managing this risk factor has become a global medical requirement which entails a thorough investigation of effective antiradical drugs. A portion of these efforts focused on plant-based compounds. Since then, several crude extracts from various parts of the plants have demonstrated a considerable antiradical activity in vitro. This property can be attributed to the specific plant-derived products which have promising free radical scavenging properties [41-42].
Table 1: Phytometabolic products and their screening tests of C. oblonga’s seeds extracts
|
UD |
UH |
UC |
UA |
UE |
Executed test |
Phytometabolites |
|
- N |
- N |
- N |
- N |
- N |
Pew’s test |
Flavonoids |
|
- N |
- N |
- N |
- N |
- N |
Lead acetate test |
|
|
+ P |
+ P |
+ P |
+ P |
+ P |
NaOH test |
Coumarins |
|
+ P |
+ P |
+ P |
+ P |
+ P |
Fluorescence test |
|
|
- N |
- N |
- N |
- N |
+ P |
Braymer’s test |
Tannins |
|
- N |
- N |
- N |
- N |
- N |
Liebermann- Burchard test |
Terpenoids |
|
- N |
- N |
- N |
+ P |
+ P |
Molish’s test |
Carbohydrates |
|
- N |
- N |
- N |
+ P |
+ P |
Mayer’s test |
Alkaloids |
|
- N |
- N |
- N |
- N |
- N |
Ammonium hydroxide test |
Emodins |
|
+P |
- N |
+ P |
+ P |
+ P |
Pigment-dependent test |
Betacyanin |
|
- N |
- N |
- N |
- N |
- N |
Pigment-dependent test |
Anthocyanin |
|
+ P |
- N |
+ P |
+ P |
+ P |
FeCl3 test |
Phenols |
|
+ P |
+ P |
+ P |
+ P |
+ P |
Xanthoproteic test |
Proteins |
|
- N |
- N |
- N |
- N |
- N |
Ninhydrine test |
Amino acids |
|
+ P |
+ P |
+ P |
+ P |
+ P |
Salkowski test |
Steroids |
|
- N |
- N |
- N |
- N |
- N |
Foam test |
Saponins
|
|
- N |
- N |
- N |
- N |
- N |
Olive oil test |
|
|
- N |
- N |
- N |
- N |
- N |
Liebermann’s test |
Glycosides |
UE, UA, UC, UD, and UH are the symbols for the ultrasonic-used solvents ethanol, ethyl acetate, chloroform, diethyl ether, and hexane, respectively.
The results recorded in Table 2 and depicted in Figure 2 indicate that the antiradical activities of crude ethanolic extract are grander than the remaining four extracts, while the n-hexane extract exhibited the lowest antioxidant activities. According to the phytochemical screening, the ethanolic extract’s preeminent antioxidant activity may be attributable to its higher overall Phyto components than the other extracts. In addition, this could be due to the plant-derived products of the moderate polarity, which are withdrawn by ethanol with higher antiradical properties [43].
Table 2: The obtained results from investigating the antioxidant potential of C. oblonga seeds’ extracts using three independent assays.
|
Symbols of reference and extracts |
SC50±SD |
||
|
DPPH-radical snaring effect |
Hydroxyl reactive moiety-snaring effect |
Total reducing effect |
|
|
Vit. C |
48.57 ± 0.84 |
50.33 ± 0.78 |
48.17 ± 0.92 |
|
UE |
106.12 ± 0.76 |
108.09 ± 0.91 |
104. 23 ± 0.87 |
|
UA |
150.46 ± 0.97 |
157.12 ± 0.83 |
148.65 ± 0.68 |
|
UC |
164.94 ± 0.88 |
161.39 ± 0.73 |
164.87 ± 0.83 |
|
UD |
292.04 ± 0.75 |
287.14 ± 0.80 |
294.11 ± 0.79 |
|
UH |
408.97 ± 0.71 |
405.65 ± 0.92 |
411.22 ± 0.78 |
SC50 was quantified in μg/mL, and every single run was implemented in three separate trials (n=3)

Figure 2: Diagrammatic depiction of the results acquired from assessing the antioxidant activity of C. oblonga seeds extracts
The valuable free radical and reactive moieties snaring potentials of C. oblonga seeds’ ethanolic extract have further been reported using extraction methods other than those employed in this study. Shaida et al. reported 299.98 μg/mL as the IC50 value of DPPH scavenging by the ethanolic extract of C. oblonga’s seeds [18]. Amin et al. investigated the antioxidant potential of the C. oblonga seeds’ ethanolic extract, finding the IC50 values of DPPH and H2O2 scavenging to be 361.68 and 144.92 μg/mL, respectively. In addition, the free radical scavenging potential of chloroform extract was also investigated, finding the IC50 values of DPPH and H2O2 scavenging to be 352.46 and 140.28 μg/mL, respectively [44]. It is worth noting that the antioxidant activity findings of our study outperform those of the preceding studies. This can be attributed to the chemical, physical, and mechanical changes generated by the ultrasonic energy, resulting in the better withdrawal of plant-derived products with radical snaring potential into the extracting media than the other extraction methods [19,43, 34].
Evaluation of Hypoglycemic Activity
Hindering the absorption of dietary carbohydrates is one of the therapeutic techniques used to manage postprandial hyperglycemia in diabetes. In the digestive tract, pancreatic α-amylase helps to break down ingested carbohydrates like starch into simple monosaccharides. After that, α-glucosidases break them down further into glucose, which is absorbed into the bloodstream. By blocking the enzymes α-amylase and α-glucosidase, carbohydrate digestion is slow down, glucose uptake is delayed, and blood sugar levels are reduced. In practice, medications like acarbose and miglitol suppress such enzymes; however, they cause many side effects, like flatulence and diarrhea [45,46].
According to this approach, and after evaluating their antioxidant activity, the capacity of the C. oblonga seeds’ extracts to inhibit porcine α-amylase and yeast α-glucosidase was assessed to determine their antidiabetic potential. The inhibitory properties of the seeds’ extract and the standard medication acarbose against α-glucosidase and α-amylase, represented as IC50 values, are indicated in Table 3 and depicted in Figure 3. The maximum inhibitory effect was detected in the standard medication acarbose, followed by ethanol, ethyl acetate, chloroform, ether, and n-hexane extracts. It was reported that many plants with various phytometabolites, like coumarins, alkaloids, and phenolics, have antidiabetic potential [47]. The ethanolic extract exhibited a higher potential for free radical scavenging and enzyme inhibition than the other solvent extracts due to its numerous phytoconstituents.
Table 3: The results obtained from investigating the antidiabetic potential of C. oblonga seeds’ extracts using three independent assays.
|
Symbols of the reference and extracts |
IC50±SD |
|
|
α-Glucosidase inhibitory impact |
α-Amylase inhibitory impact |
|
|
Acarbose |
284.16 ± 0.67 |
265.34 ± 0.70 |
|
UE |
391.28 ± 0.72 |
383.68 ± 0.76 |
|
UA |
486.02 ± 0.65 |
470.46 ± 0.65 |
|
UC |
486.16 ± 0.76 |
480.01 ± 0.69 |
|
UD |
711.09 ± 0.59 |
702.93 ± 0.72 |
|
UH |
737.62 ± 0.68 |
726.12 ± 0.71 |
IC50 was quantified in μg/mL, and each run was implemented in three separate trials (n=3).
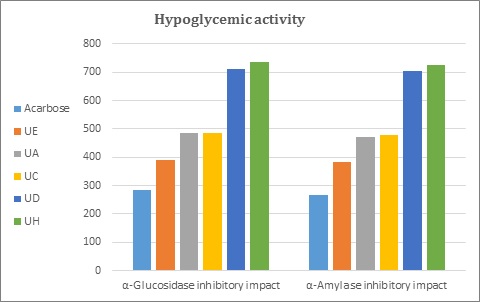
Figure 3: Diagrammatic depiction of the results obtained from assessing the antidiabetic activity of C. oblonga seeds’ extracts
Conclusion
The results of this study revealed that the sonication-acquired extracts of C. oblonga’s seeds are good sources of key phytochemical components and have significant antioxidant and antidiabetic properties. Our findings demonstrated that ethanolic extract is a more effective antioxidant than other solvent extracts. In addition, a marked antidiabetic activity was reported by the crude extracts, particularly the ethanolic one. Several investigations have confirmed the function of free radicals in the development and progression of diabetes. As a result, compounds with antidiabetic and antioxidant properties that do not cause serious side effects will be advantageous. Based on these findings, C. oblonga’s seeds can be considered a promising source of the lead compounds for diabetes prevention, therapy, or both. Doubtless, this will motivate more research into the hypoglycemic potential of this plant.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
Acknowledgments
The authors are praising the University of Mosul/College of Pharmacy for providing facilities that improved the quality of this work.
ORCID:
Raghad Riyadh Khalil
https://www.orcid.org/0000-0001-7682-9278
Eman Tareq Mohammed
https://www.orcid.org/0000-0002-2350-2164
Yasser Fakri Mustafa
https://www.orcid.org/0000-0002-0926-7428
HOW TO CITE THIS ARTICLE
Raghad Riyadh Khalil, Eman Tareq Mohammed, Yasser Fakri Mustafa, Evaluation of In vitro Antioxidant and Antidiabetic Properties of Cydonia Oblonga Seeds' Extracts, J. Med. Chem. Sci., 2022, 5(6) 1048-1058


