Document Type : Original Article
Authors
Department of Chemistry, College of Science, University of Thi-Qar, Iraq
Abstract
This study reports a comparison between two types of extraction. The first one is traditional extraction which can be called liquid-liquid extraction (LLE) or solvent extraction, while the second is modern extraction, namely cloud point extraction (CPE). 3-Chloro-2,4-pentanedione was used as a complexing agent that is one of β-diketone that have an interesting in extracting copper ions. The current study showed that cloud point extraction (E%=92, D=11.594, LogD= 1.064233296) is more efficient than its counterpart (E%=80, D=4.089, LogD= 0.611). The cloud point extraction was studied using Triton X-100 as a non-ionic surfactant and ethyl acetate as a solvent. Also, complex CuA-Cl was prepared and characterized by elemental analysis and mass and UV-visible spectrometries.
Graphical Abstract
Keywords
Main Subjects
Introduction
Copper is widely distributed in nature and is a nutritionally essential element. There is a need to develop sensitive, inexpensive, and selective methods for the determination of copper in biological and environmental individuals [1]. Solvent extraction was used in 1986 to extract copper from dilute leach liquors for dump leach operations. It was used again for the Chelating extraction of copper for the first time in 1959, using one of the β-diketone compounds (acetylacetone). Then successive use of other β-diketone derivatives such as butyl acetylacetone, allyl acetylacetone, benzoyl acetone, dibenzoyl methane, thenoyltrifluoroacetone, furoyltrifluoroacetone [2- 5], While LIX ® 54 (1-phenyldecanone-1,3-diones) was used with CPE for extract copper from ammoniacal media.
Metal β-diketonates are the earliest metal compounds and played an essential role in coordination chemistry. Also, the complexes of metals have been used extensively in analytical chemistry due to the ease of extraction of these compounds. β-Diketones complexes have been used in science and industry as polymerization catalysts, UV resistance, oxygen resistance, and pharmaceutical and cosmetic ingredients. Furthermore, β-diketones have been used in environmental protection due to their complexing properties for metal chelation in sewage [2,6-7].
Solvent extraction is one of the oldest techniques for purification, sample preparation, extraction of the component from the matrix. The solvent extraction process depends on the two immiscible solvents, one of which contains the dissolved component (the solute), and that solvent is called the carrier. However, the dissolved component (the solute) extracts into extracted solvent from the carrier, and the remaining solution from this extraction is called Raffinate [8-12]. Solvent extraction is slow and environmentally unfriendly due to using large solvents. Therefore, there was a need to develop extraction procedures, as in the case of CPE [13].
CPE is a sensitive extraction, separation, pre-concentration procedure based on the formation of a cloud point during extraction by micelle formation after using a surfactant and an appropriate temperature. CPE is an essential analytical, green and environmental chemistry technique for discovering trace metals in matrices [14].
In general, the procedure is based on the following strategy, the surfactant-rich phase forms and separates from the aqueous phase if the condition includes using an appropriate surfactant, temperature, and pressure. The metal is captured from micelle's bulk solution, which may be associated with a ligand [15].
To compare solvent extraction and CPE. CPE, it is undoubtedly that solvent extraction is extraction and pre-concentration method, but have some disadvantages. The organic solvent in the solvent extraction method is toxic, of high cost, highly flammable, and environmentally unfriendly. For example, these solvents are alcohols, esters, ethers, ketones, and aldehyde. Therefore, there is a trend to replace it with the green extraction method. Sometimes solvent extraction has advantages such as low energy input (evaporation and distillation), the solvent can be recycled, and has selectivity [16-18].
On the other hand, CPE is an alternative green method for solvent extraction. Using surfactants (also named surface-active agents) gives the CPE advantages due to their capacity to dissolve many chemicals with different natures and are poorly soluble in water. CPE is non-toxic, simple, and highly capable of concentrating the analytes. For example, these surfactants are Triton X-100, Triton X-114, Tween 20, Tween 80, and Tegitol TMN 6 [14,19].
The current study will investigate the comparison between liquid-liquid extraction (LLE) or solvent extraction and cloud point extraction (CPE) in extraction of copper ion using 3-chloro-2, 4-pentandione.
Martials and Methods
Chemicals
3-Chloro-2,4-pentanedione was obtained from Hyper chem/ China. The nonionic surfactant Triton X-114 was obtained from Sigma Alidrich. A stock solution of Cu (II) was prepared from CuSO4.5H2O (BDH) at concentration 100 µ/ml. Hydrochloric acid and sodium hydroxide were obtained from BDH, England respectively. The solvent Ethyl acetate was from BDH. All chemical were used without further purification.
Synthesis of copper complex
Dissolve (0.804 g, 4.29 mol) of copper acetate in the minimum amount of solvent (1:1 methanol: water). Over 15 minutes with stirring, add a solution of (1 ml, 8.85 mol) 3-chloro-2,4-pentanedione in the least volume of methanol. Heat the reaction to 80 °C for 2 hours, followed by cooling in an ice bath. Filter the precipitate using a filtration funnel, wash with cold distilled water, and then methanol, dry under vacuum to afford the greenish-grey precipitate (Figure 1), yield (1.42 g, 97%). [29] Elemental analysis (%) calculated for CuA-Cl: C 36.33, H 3.66; found: C 31.324, H 3.251. MS (m/z): 331.2[M]•+, 295[M-Cl], 231.1[M-100.1], 215[M-116.2], 201[M-130], 134.56[M-197], 119.52[M-211.68], 43[C2H3O+].

Figure 1: Synthesis of complex CuA-Cl
Physical properties and the yield
CuA-Cl is a greenish-grey precipitate, melt at 273 °C and soluble in Acetonitrile, Chloroform, Methanol, Ethanol, Acetone, Ethyl acetate, DCM, and DMF but is insoluble in Ether, Hexane, and Benzene, and partially dissolved in water. The yield of the reaction was 97%.
Absorption spectrum of copper complex
The UV-Visible absorption spectrum of CuA-Cl complex in ethanol was recorded using a T90+ UV-Visible spectrometer (PG Instruments Ltd), a conventional quartz cell having an optical path length of 1 cm. In Figure 2, an illustration of the complex spectrum was performed at a concentration of 0.05 mM and in the range of wavelength (200-900) nm.

Figure 2: UV-Visible spectrum of CuA-Cl in concentration 0.05 mM at ethanol
Calibration curve for determination of copper ion
Typically, a set of standard solutions containing CuA-Cl complex was prepared from a stock solution of 100 µg. ml-1. The absorption was recorded for these solutions and graph calibration as in Figure 3.

Figure 3: Calibration curve of CuA-Cl complex at ethanol (327 nm)
Procedure for solvent extraction
The solvent extraction was carried out by dissolving an appropriate volume of 3-Chloro-2,4-pentanedione in an appropriate volume of Ethyl acetate. An aliquot of 10 ml of an aqueous solution containing Copper(II) sulfate pentahydrate at the desired pH and ethyl acetate containing 3-Chloro-2,4-pentanedione were introduced into a glass test tube. The pH was adjusted using concentrated hydrochloric acid or sodium hydroxide. The solution into the test tube was mixed for various minutes using a mix-shaker model (Jeio tech Bs-11, Korea) and state for 15 minutes and that at various temperatures. The downer aqueous phase was removed and then determined by a UV-visible spectrophotometer. [30]
Procedure for cloud point extraction
The cloud point extraction was carried out by dissolving an appropriate volume of 3-Chloro-2,4-pentanedione in 1 ml of Triton X-100. An aliquot of 10 ml of an aqueous solution containing Copper(II) sulfate pentahydrate at the desired pH and Triton X-100 containing 3-Chloro-2,4-pentanedione were introduced into a glass test tube. The pH was adjusted using concentrated hydrochloric acid or sodium hydroxide. The solution into the test tube was mixed for various minutes using a mix-shaker model (Jeio tech Bs-11, Korea) and state for 15 minutes and that at various temperatures. The upper aqueous phase was removed and then determined by a UV-visible spectrophotometer. [19,31]
Results and Discussion
Two of 3-chloro-2,4-pentanedione are coordinated with copper ion (II) to afford the bis(3-chloro-acetylacetonato) copper (II), CuA-Cl. The maximum wavelength at which the concentrations are determined was selected from the UV-visible spectrum of CuA-Cl. To reach the best results in the extraction processes, the effects of different parameters such as the amount of the complexing agent, solution pH, organic phase volume, time (incubation time in CPE), and temperature were optimized. The optimization strategy was used to optimize those parameters.
Distribution coefficient
Typically, extraction uses two immiscible solvents. One solvent is polar such as water, and another is non-polar such as hexane, and benzene. The neutral species could be extracted into the non-polar solvent. To follow the extraction processes, two parameters could be calculated. The partition coefficient Kp is the more fundamental one which expresses the concentration of species in both solvents as equation 1:
Whereas
Kp: the partition coefficient, : the concentration of unionized species in the organic solvent, : the concentration of unionized species in the aqueous solvent.
A more practical parameter is the Distribution ratio D, which represents the ratio of the total analytical concentration in both solvents as the following equation:
D: Distribution ratio, : the concentration of unionized and ionized species in the organic solvent, : the concentration of unionized and ionized species in the aqueous solvent [17,18].
Effect of amount of the complexing agent (3-chloro-2, 4-pentanedione)
In solvent extraction, the effect of 3-Chloro-2,4-pentanedione as the complexing agent on the distribution ratio of extraction Cu(II) was studied in a volume range of (0.5-3) µL. The results showed that the distribution ratio increased up to 1 µL, then decreased. The volume 1 µL was chosen as the optimum volume in this study. In cloud point extraction, the optimum volume was the same as in solvent extraction. However, the distribution ratios in cloud point extraction were higher than in its counterpart (Table 1 and Figure 4). It can be concluded that a volume of 1 µL of complexing agent provides more sensitivity and a more stable ion-pair complex extracted by both cloud point and solvent procedures [20].

Effect of solution pH
The solution pH plays an essential role in forming complex and the subsequent extraction. Enol-keto tautomerization of 3-Chloro-2,4-pentanedione depends on pH strongly. However, increasing pH increases the enol form and thus the complexation reaction [21,22]. Besides, the pH effect on cloud point extraction depends on the characteristics of nonionic surfactant Triton X-100. Where, the critical micelle concentration decrease when pH increase. The enol form in the micellar phase is more stable due to the low polarity of surfactant [23,24] (Figure 5).
In the current study, the distribution ratio increase when the pH increase in both procedures. Thus, pH 9 was chosen as the optimum pH in the subsequent experiments, see Table 2 and Figure 6. Also, the distribution ratios in cloud point extraction were higher than in solvent extraction.

Effect of volume of organic and pseudo-organic phase
In solvent extraction, the results in Figure 6 illustrate that the extraction efficiency increases with an increase in an organic phase volume. Thus, 35 ml of ethyl acetate was used as the optimum organic phase volume for subsequent experiments. The extraction efficiency in CPE increases when the volume of the surfactant increases from 0.5 ml to 1 mL, and then begins to decrease. To explain these, the volume of the surfactant plays an important role in the separation of phases. In a lower volume of the surfactant, we get an incomplete phase separation obtained, but when the volume increases, it leads to CMC. When the volume is increased more, this leads to an increase in the viscosity and thus a decrease in the extraction efficiency. Thus, 1 mL of Triton X-100 is the optimum volume of surfactant for subsequent experiments [25,26] (Table 3 and Figure 7). In the third extraction experiment, it was found that the distribution ratios for solvent extraction are lower than for cloud point extraction.

Figure 6: Effect of solution pH on extraction Cu (II) by (A) solvent extraction (B) cloud point extractionEffect of time
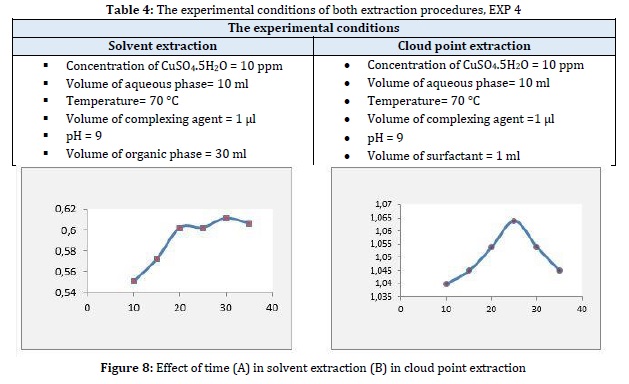
Solvent extraction is an equilibrium procedure, and the maximum amount can be extracted after equilibrium is obtained. The extraction efficiency increased with increasing extraction time from 10 to 30 min and then no increase in 35 minutes was observed. Therefore, a time of 30 min was chosen for subsequent experiments. The range of 10 to 35 min was chosen to study the influence of incubation time in CPE. Whereas, the extraction efficiency increased up to 25, and then it decreased. The separation of the surfactant-rich phase was higher at 25 min and less or incomplete at the other times. Therefore, a time of 25 min was chosen for subsequent experiments, see Table 4 and Figure 8. It was noticed that the distribution ratios in solvent extraction are also lower [27].
Effect of temperature
Solvent extraction was studied at different temperatures ranging from 50 to 70 °C. The extraction efficiency has significantly increased with increasing temperature. Probably, the heating improved the extraction efficiency due to increased 3-Chloro-2,4-pentanedione solubility and complexation reaction between a metal ion and complexing agent. Undoubtedly, the issue in CPE is a little different from solvent extraction. The ranging heat was from 70 to 90 °C. Theoretically, CPE could have occurred when the temperature of extraction is higher than the CPT (cloud point temperature). In lower temperatures, the micellar and aqueous phases separation cant occur while in higher temperatures, which will lead to a breakdown of hydrogen bond in the surfactant-rich phase. The optimal equilibrium temperature of CPE occurs at 80 °C higher than the CPE temperature of the surfactant [28] (Table 5 and Figure 9). The last experiment found that the distribution ratios for solvent extraction are lower than the distribution ratios for cloud point extraction.

Conclusions
The comparison between the solvent procedure and the cloud point procedure showed a significant difference, as the cloud point procedure was more efficient with a higher extraction percentage. Such differences between them may result from the combination of various phenomena, including effects of surfactant, solvent, and effects differences temperature on both substances above and interaction between complexing agent and formed a complex with two systems (surfactant or solvent). The high values of extraction percentage in both procedures indicated the strength of the 3-Chloro-2,4-pentanedione as a complexing agent.
The findings of this study show that the optimum conditions for solvent extraction were 1µl of complexing agent, pH equal to 9 at 30 mL of ethyl acetate as a solvent, throughout 35 min (E%=80, D=4.089, LogD= 0.611). Similarly, the optimum conditions that could point extraction were 1µL of complexing agent, pH equal to 9 while the volume of Triton X-100 as a surfactant was 1 ml, and the time was 25 min (E%=92, D=11.594, LogD= 1.064233296).
Acknowledgment
The Acknowledgments should be placed in a separate paragraph after the Experimental section. Technical assistance, material support, and other help or advice may be acknowledged briefly in this section (Font: Time New Roman 10).
Funding
This research did not receive any specific grant from fundig agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to responsible for all the aspects of this work.
Conflict of Interest
The authors have no conflicts of interest relevant to this article.
HOW TO CITE THIS ARTICLE
Azhar Hameed Gatea, Saher A. Ali Alshamkhawy, Wathiq Sattar Abdul-hassan. Comparison Study of Cloud Point and Solvent Extraction of Copper by 3-Chloro-2,4-pentanedione as Complexing Agent, J. Med. Chem. Sci., 2022, 5(5) 743-752
- Jamshid M.L., Ghasem K.N., J. Chem. Chem. Eng., 2005, 24:47 [Publisher]
- Stary J., The solvent extraction of metal chelates, the Pitman Press, Bath, Great Britain, 1964 [Google Scholar], [Publisher]
- Dalton R.F., Severs K.J., Stephens G., Mining Latin America / Minería Latinoamericana, 1986, 67 [Crossref] [Google Scholar] [Publisher]
- Radzyminska-Lenarcik E., Witt K., Sci. Technol., 2018, 53:1223 [Crossref], [Google Scholar], [Publisher]
- Casey R.J., Fardy J.J.M., Walker W.R., Inorg. Nucl. Chem., 1967, 29:1139 [Crossref] [Google Scholar] [Publisher]
- Zhang Q.W., Lin L.G., Ye W.C., Med., 2018, 13:1 [Crossref] [Google Scholar] [Publisher]
- Urbaniak K., Jurek K., Witt K., Gorączko A., Chemik. 2011, 65:273 [Google Scholar] [Publisher]
- Patel K., Panchal N., Ingle P., J. Adv. Res. Chem. Sci., 2019, 6:6 [Crossref] [Google Scholar] [Publisher]
- Zhang Q.W., Lin L.G., Ye W.C., Med., 2018, 6:6 [Crossref] [Google Scholar] [Publisher]
- Aguilar M., Cortina J.L., Solvent extraction and liquid membranes: Fundamentals and applications in new materials, Taylor & Francis, 2008 [Google Scholar], [Publisher]
- Alper E., Introduction to Liquid-Liquid Extraction with Chemical Reaction, Springer, Dordrecht, 1983 [Crossref], [Google Scholar], [Publisher]
- Scoppola E., Solvent extraction: a study of the liquid/liquid interface with ligands combining x-ray and neutron reflectivity measurements, Doctoral dissertation, Université Montpellier, 2019 [Google Scholar], [Publisher]
- Zurich, IPE Separation Process Laboratory, ETH Zurich, 2014, 6:1 [Publisher]
- Bezerra M.A., Arruda M.A., Ferreira S.L.C., Spectrosc. Rev., 2005, 40:269 [Crossref] [Google Scholar] [Publisher]
- Ingram T., Storm S., Glembin P., Bendt S., Huber D., Mehling T., Smirnova I., Ing. Tech., 2012, 84:840 [Crossref] [Google Scholar] [Publisher]
- Płotka-Wasylka J., Rutkowska M., Owczarek K., Tobiszewski M., Namiesnik J., TrAC Trends Analyt. Chem., 2017, 91:12 [Crossref] [Google Scholar] [Publisher]
- Kealey D., Talanta, 1974, 21:475 [Crossref], [Google Scholar], [Publisher]
- Berthod A., Carda-Broch S., Chromatogr. A., 2004, 1037:3 [Crossref] [Google Scholar] [Publisher]
- Favre-Reguillon A., Murat D., Cote G., Draye M., Chem. Technol. Biotechnol.,. 2012, 87:1497 [Crossref] [Google Scholar] [Publisher]
- Kori S., Forensic Sci. Res., 2021, 6:19 [Crossref] [Google Scholar] [Publisher]
- Levie R., Advanced Excel for scientific data analysis, Oxford University Press, USA, 2004, 182 [Google Scholar], [Publisher]
- Sanna D., Varnagy K., Timari S., Micera G., Garribba E., Chem., 2011, 50:10328 [Crossref] [Google Scholar] [Publisher]
- Bhatia N.K., Kishor S., Katyal N., Gogoi P., Narang P., Deep S., RSC Adv., 2016, 6:103275 [Crossref] [Google Scholar] [Publisher]
- Bloor J.R., Morrison J.C., Rhodes C.T., Pharm. Sci., 1970, 59:387 [Crossref] [Google Scholar] [Publisher]
- Wang C., Martin D.F., Martin B., Environ. Sci. Health, 1996, 31:1101 [Crossref] [Google Scholar] [Publisher]
- Motikar P.D., More P.R., Arya S.S., Sci. Technol., 2021, 56:1014 [Crossref] [Google Scholar] [Publisher]
- Yang X., Jia Z., Yang X., Li G., Liao X., Saudi J. Boil. Sci., 2017, 24:589 [Crossref] [Google Scholar] [Publisher]
- Noorashikin M.D.S., Sohaimi N.M., Suda N., Sustain. Sci. Manag., 2017, 12:79 [Google Scholar] [Publisher]
- Magritek, AZO MATERIALS, 2015 [Crossref], [Google Scholar], [Publisher]
- Bermejo J.C., Alonso M., Sastre A.M., Alguacil F.J., Chem. Res., 2000, 10:479 [Crossref] [Google Scholar] [Publisher]
- Ferrera Z.S., Sanz C.P., Santana J.J., Rodriguez, TrAC Trends Analyt. Chem., 2004, 23:469 [Crossref] [Google Scholar] [Publisher]


