Document Type : Original Article (Special Issue)
Author
Department of Medical Laboratory Techniques, Al-Mussaib Technical Institute, Al-Furat Al-Awsat Technical University, 51009, Iraq
Abstract
Breast cancer is the first among the top ten cancers as a significant public health issue for women in Iraq. The study was conducted to investigate the association of estrogen and progesterone risks in breast cancer. This study contained sixty paraffin blocks divided into 40 samples of breast cancer blocks and 20 samples of normal breast tissues to examine the expression of estrogen receptors and progesterone receptors in Iraqi patients with breast cancer. The samples were immunohistochemical examined with Dako kits (Dako, Denmark), with higher scores suggesting the presence and density of these receptors (ER and PR). According to the findings of this investigation, the majority of patients were tested positive for ER and PR receptors. There is increasing evidence that ER and PR receptors have a role in the development of breast cancer, making it a desirable protein to become a key target for cancer treatment.
Graphical Abstract
Keywords
Introduction
During the last three decades, breast cancer has become more prevalent in Iraq, ranking as the second-highest cause of death after cerebrovascular diseases. This poses a significant problem for the public's health, particularly with regard to women's health [1,2].
Unfortunately, many Iraqi patients are already in the advanced stages of their conditions when they are first identified with it [3]. Breast tumors can be classified into four molecular subtypes based on gene expression patterns (luminal A, luminal B, HER2 (human epidermal growth factor receptor 2)-enriched, and basal-like) [4,5]. The classification of HER2 and Ki-67 (marker of proliferation Ki-67) in clinical practice using immunohistochemistry and/or fluorescence in situ hybridism has been simplified as a result of the finding of the estrogen receptor (ER) and the progesterone receptor (PR) [6].
The classification outcome aids in decision-making. According to the previous research, HR+HER2+ breast cancer had an improved prognosis than HR-HER2+ breast cancer [7]. Unlike IHC substitution classification, intrinsic molecular classification based on gene expression profiling may expose the molecular essence and propose therapeutic sensitivity (8,9,10). Because traditional clinical characteristics such as type, stage, and disease grade are no longer sufficient, researchers have identified four primary forms of breast cancer based on the estrogen (ER), progesterone (PR), and epidermal growth factor receptor 2 (HER2) receptors that are present in the body [11].
At the protein level of breast cancer, non-specific breast cancer indicators such as 15-3 carcinoembryonic antigen (CA) and 27.29 have been discovered as promising biomarkers through histological and biochemical research [12].
Women who have the early menarche and bilateral ovariectomy before the age of 45 years old have the early natural menopause, according to the population rates before and after menopause [13].
Premenopausal plasma levels of breast and sex steroid hormones have generated mixed outcomes in epidemiological studies. Premenopausal estradiol levels in the blood and the risk of breast cancer are only the subjects of five forthcoming investigations [14].
The study was conducted to investigate the association of estrogen and progesterone risks in breast cancer.
Material and Methods
The Oncology Teaching Hospital (OTH) and the National Cancer Research Center (NCRC) in Baghdad conducted assessments on 80 women diagnosed with breast cancer. The tissue sample is initially fixed in 10% formaldehyde, and then sectioned and stained with Hematoxylin and Eosin dyes for histological investigation. Formalin-fixed, paraffin-embedded blocks were analyzed using immunohistochemistry (IHC) for the presence of hormone receptor (ER, PR) expression. Primary tumors were assessed by using the Allred scoring system based on staining intensity and percentages of positive tumor cells.
In the case of both ER and PR, the tumor size should be less than 3. There are three levels 2 of positivity, with scores 3–4, 5–6, and 7–8 representing the three levels. Higher scores showed the availability and abundance of these receptors when utilizing Dako kits (Dako, Denmark). To be considered affirmative, the staining reactions had to be seen in at least 10% of the malignant cells. The staining strength was assessed as strong (+3), medium (+2), or weak (+1). It was shown that 30% of malignant cells had a total black membrane staining that was positive and graded (+3) by HER2 testing. The equivocal staining reactions and/or heterogeneous staining distribution in less than 30% of cells were evaluated as (+2) in cases with thin membrane dubious staining reactions.
The results of fluorescence and chromogenic in situ hybridization (CISH) studies on tissue sections from the same initial tumor later characterized the latter as positive or negative, respectively. When ER/PR and HER2 are both negative, the analyzed breast cancers were divided into four molecular subtypes: Luminal A when both are positive, Luminal B when both are negative, Non-Luminal/HER-2-enriched when both are positive, and Non-Luminal/Triple Negative when both are negative.
Results
It was revealed that 14 of the 35 women with ER/PR+ initial findings were ER+/PR+, 9 had the same results, and 12 had the same outcomes as ER+/PR+. In our seven re-evaluated and confirmed ER/PR+ instances, the staining proportions of malignant cells were 0% in the ER and 1–69 percent (average=15.8 percent) in the PR. When the ER/PR+ diagnosis was revisited, it was found that the average percentage of PR staining had been decreased. The small number of cases in the study likely explains why no significant relationship was discovered between ER, PR, and age. On the other hand, the kind of breast cancer was linked to ER and PR. No correlation was found between the histological grades of breast cancer and progesterone or estrogen receptor status.
The methodology was the same in ER and PR. The re-evaluated ER, PR, and H&E slides are displayed in (Figures 1 and 2).
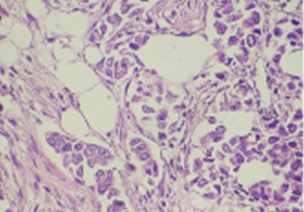
Figure 1: Showed breast cancer tissue staining with H&E (40X)

Figure 2: Showed negative staining breast cancer tissue (40X)
The PR positive staining in tumor cells was detected in 23/35 (91%) cases of BC, 16 cases with moderate intensity coloring and 7 cases with weak intensity coloring, and 12/35 (34%) cases with negative results for PR (Figure 3 and 4). ER expression was observed in tumor cells in 20/35 (57%) cases of BC, 14 cases with strong intensity coloring and 6 cases with moderate intensity coloring, and 15/35 (42.8%) cases with negative results for ER (Figures 5 and 6).
Discussion
Core needle biopsy specimens are the subject of the immunohistochemical Iraq research, which examines the association between clinical and pathological data. Age-related ER/PR expression changes have been seen in Western countries and Iraq [15]. More than half of the patients in the Iraq research had cancers of the estrogen and progesterone receptors (ER and PR), as well as HER2 [16].
Cancer was diagnosed in the sixth lifetime of those patients at the time of diagnosis, according to an Iraq study that presented the clinical characteristics of patients with a family history of breast cancer [17].

Figure 3: Showed weak staining for progesterone receptors (PR) breast cancer tissue in nuclei (40X)
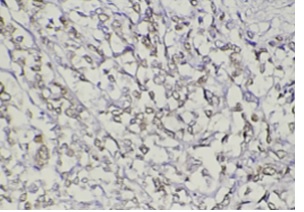
Figure 4: Showed moderate staining for progesterone receptors (PR) breast cancer tissue in nuclei (40X)

Figure 5: Showed moderate staining for estrogen receptors (ER) breast cancer tissue in nuclei and cytoplasm (40X)

Figure 6: Showed strong staining for estrogen receptors (ER) breast cancer tissue in nuclei and cytoplasm (40X)
Androgens may play a role in breast cancer formation, although experimental evidence reveals that androgens have both stimulating and inhibiting effects on in vitro mammary epithelium and cancer cell growth, as well as on experimentally generated mammary tumor growth [18].
It is possible to find steroidogenic enzymes in both normal breast tissue and breast cancer specimens; however, high serum androgen levels may lead to increase estrogenic exposures in the breast tissue. This is due to the fact that it is possible to find steroidogenic enzymes in both normal breast tissue and breast cancer specimens [19]. Nadji et al. performed an IHC study on 5993 breast carcinomas that tested positive for the estrogen receptor. The ER-/PgR+ phenotype was not discovered [20].
De Maeyer et al. found that none of the 32 cases previously thought to be ER/PgR+ were discovered after a re-evaluation with IHC [21]. According to H. et al. (2019), of the 267 cases studied in Kuroda, 92 were verified as ER/PR+ [22].
The diagnostic criteria or procedures used to prepare the IHC can be utilized to establish the discrepancies in the prior publications. Over time, the recommended IHC cut-off positions have been shifted. For both ER and PR status, Ogawa et al. found that a stained percentage of 10% may be an appropriate cut-off value for IHC [23].
The increased risk of breast cancer can result from longer lifetime exposure to reproductive hormones. Furthermore, the risk occurs closer to HR+ breast cancer than to the other subtypes [24]. Postmenopausal women with naturally elevated levels of some endogenous sex hormones (e.g., estrogen, progesterone) have around twice the risk of developing breast cancer relative to those with the lowest levels, with the highest association observed among HR+ tumors [25].
Conclusion
Immunohistochemical labeling for hormone receptor expression in breast cancer tissues is commonly used to determine tumor aggressiveness and the necessity of additional neoadjuvant therapy. Both ER and PR had strong associations with the forms of breast cancer found in the study, while histological grade of breast cancer did not affect the expression of ER and PR.
Acknowledgment
The authors thank the Oncology Teaching Hospital in the Medical City in Baghdad and the National Cancer Center for their participation to make this study possible.
Funding
This research did not receive any specific grant from fundig agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
There are no conflicts of interest in this study.
ORCID:
Ruqayah Ali Salman
https://www.orcid.org/0000-0002-1750-0576
HOW TO CITE THIS ARTICLE
Ruqayah Ali Salman. Immunohistochemical Determination of Estrogen and Progesterone Receptors In Women Breast Cancer Patients. J. Med. Chem. Sci., 2022, 5(7) 1224-1230


